Table
Return to top.
Figure
Return to top.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5144a1.htm
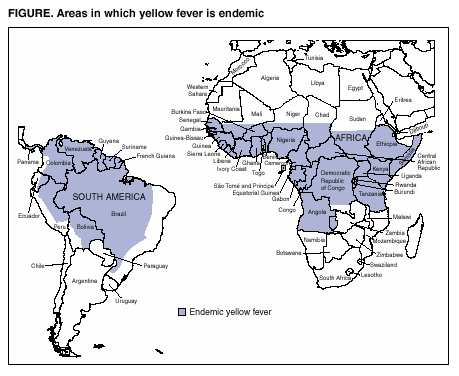
In June 2001, seven cases of yellow fever vaccine--associated viscerotropic disease (YEL-AVD) (previously called multiple organ system failure) in recipients of 17D-derived yellow fever vaccine (YEL) were reported to the Advisory Committee on Immunization Practices (ACIP) (1--3). ACIP reviewed the cases, recommended enhanced surveillance for adverse events, and updated the ACIP statement on YEL (4). This report summarizes the preliminary surveillance findings, including two new suspected cases of YEL-AVD and four suspected cases of YEL-associated neurotropic disease (YEL-AND) (previously called postvaccinal encephalitis). Although YEL remains essential for travelers to areas in which yellow fever (YF) is endemic (Figure), these findings underscore the need for continued enhanced surveillance and timely clinical assessment of YEL-associated disease.
The Vaccine Adverse Event Reporting System (VAERS) receives reports of adverse events following licensed vaccine administration in the United States (5). Enhanced surveillance for YEL adverse events was initiated in June 2001 and includes soliciting reports from health-care providers at certified YF-vaccination clinics and reviewing all VAERS case reports of febrile illness associated temporally with YEL (i.e., illness onset <30 days following receipt of YEL). During June 20, 2001--August 31, 2002, a total of 117 reports of adverse events following YEL administration were reported compared with 104 reports during a comparable period in 2000--2001. Of the 117 reports, six cases of persons with severe adverse events consistent with YEL-AND or YEL-AVD were reported. All six patients were vaccinated in the United States with 17D-derived YEL, required hospitalization, and recovered without sequelae. The first case was reported initially as nonserious in May 2001 but was reclassified after the enhanced surveillance system was in place.
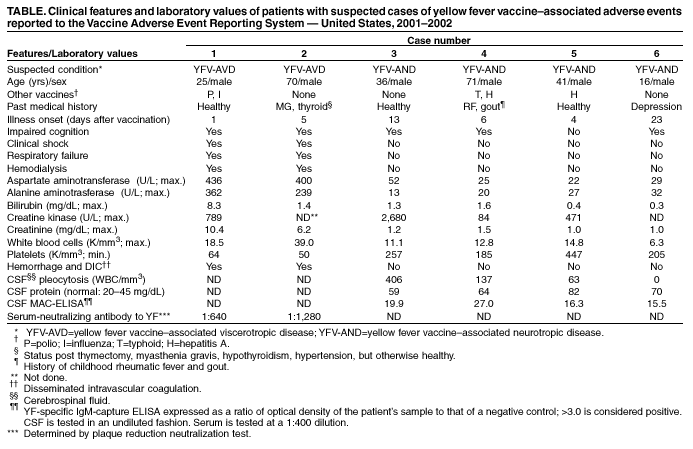
Case 1. On April 27, 2001, a man aged 25 years received YEL and influenza and poliovirus vaccines in preparation for travel to North Africa, Israel, Turkey, and Ecuador. One day after vaccination, he had lymphadenopathy, headache, and malaise; 2 days later, he reported nausea, diarrhea, diaphoresis, and fever. Nine days after vaccination, he was hospitalized with a fulminant illness characterized by fever of 101.6º F (38.7º C) and acute hepatic and renal failure (Table). The next day, he had hypotension and respiratory failure requiring resuscitation, vasopressors, dialysis, and mechanical ventilation. No bacterial pathogens were identified from urine, blood, or stool specimens. A toxicology screen was negative. After 24 days of hospitalization, he recovered and was discharged. No acute-phase serum or tissue samples for viral isolation or polymerase chain reaction (PCR) were obtained. Convalescent-phase serum samples collected 351 days after vaccination demonstrated a YF-neutralizing antibody titer of 1:640.
Case 2. On March 28, 2002, a man aged 70 years received YEL in preparation for travel to Venezuela. He had fever, dyspnea, myalgia, and malaise 5 days after vaccination; 3 days later, he was hospitalized because of fever, thrombocytopenia, and elevated hepatocellular enzymes, bilirubin, and creatinine (Table). He subsequently became hypotensive and was intubated for respiratory failure. Hyponatremia developed and dialysis was required for renal failure. Blood and urine cultures were negative for bacteria, fungi, and viruses. Serum collected on hospital days 21, 25, and 33 and pleural fluid collected on day 26 were negative by real-time, quantitative PCR (TaqMan®) with consensus flavivirus primers and viral culture. Serum collected on hospital day 26 had a neutralizing antibody titer of 1:1,280. After a 41-day hospitalization, he recovered and was discharged.
Case 3. On September 17, 2001, a man aged 36 years received YEL in preparation for travel to Brazil. He had diaphoresis, fever of 102.2º F (39.0º C), rigors, and headache 13 days after vaccination; 16 days after vaccination, he lost consciousness and was hospitalized with severe headache and fever of 106.0º F (41.1º C) (Table). Examination of cerebrospinal fluid (CSF) revealed 406 white blood cells per mm3 (WBC/mm3) (predominantly lymphocytes) and elevated protein. Blood, urine, and CSF cultures were negative for bacteria, fungi, and viruses. YF-specific IgM-capture ELISA (MAC-ELISA) of CSF was strongly positive (Table). CSF viral testing by TaqMan® and viral culture was negative. Additional MAC-ELISA results were negative for Eastern equine encephalitis, St. Louis encephalitis, West Nile encephalitis, and La Crosse encephalitis viruses. After a 5-day hospitalization, he recovered and was discharged.
Case 4. On October 4, 2001, a man aged 71 years received YEL and typhoid and hepatitis A vaccines in preparation for travel to Guatemala. He had fever and malaise 6 days later; 13 days after vaccination, he became confused, had expressive aphasia, and was hospitalized with fever of 101.1º F (38.4º C). He had leukocytosis but normal hepatocellular enzymes. CSF had 137 WBC/mm3 and elevated protein. CSF YF-specific IgM testing by MAC-ELISA was positive (Table); viral testing by TaqMan® and viral culture was negative. CSF was negative for herpes viruses, flaviviruses, and enteroviruses. After a 7-day hospitalization, he recovered and was discharged.
Case 5. On February 7, 2002, a man aged 41 years received YEL and hepatitis A vaccine in preparation for travel to Venezuela. Six days after vaccination, he had low-grade fever, headache, and myalgia, which worsened over several days; 16 days after vaccination, he was hospitalized with fever of 104.0º F (40.0º C), headache, and rigors. CSF had 63 WBC/mm3 (predominantly mononuclear) and elevated protein. Hepatocellular enzymes were normal (Table). Bacterial and fungal cultures of blood and CSF and CSF cryptococcal antigen were negative. CSF enteroviral testing and Leptospira serology were negative. CSF YF-specific IgM testing by MAC-ELISA was strongly positive (Table); viral testing by TaqMan® and viral culture was negative. After 5 days, he recovered and was discharged.
Case 6. On May 17, 2002, a boy aged 16 years received YEL in preparation for travel to South America; 23 days after vaccination, he had left-arm numbness, inability to speak, loss of right-side fine motor control, expressive aphasia, and severe dysarthria. Magnetic resonance imaging showed diffuse, bilateral, white-matter disease; CSF examination was normal. MAC-ELISA YF-specific IgM tests on CSF collected 26 days after vaccination were strongly positive (Table); CSF tests by TaqMan® with consensus flavivirus primers and viral cell culture were negative. Tests for Rocky Mountain spotted fever, herpes simplex, multiple sclerosis, lupus, autoimmune diseases, and metabolic enzyme deficiencies were negative. Reverse-transcriptase PCR with primers for Colorado tick fever was negative; serum collected 4 months after illness onset did not contain neutralizing antibodies for that virus. No bacteria or fungi were cultured from CSF. The patient was afebrile throughout his illness and was discharged after a 3-day hospitalization.
Reported by: S Levy, MD, Saint Agnes Medical Center, Fresno, California. K Mullane, DO, Loyola Univ Medical Center, Maywood, Illinois. M Miller, MD, Albany Medical College; S Siva, MD, Albany Medical Center Hospital, Albany, New York. D Barnes, MD, Southview Medical Group, Birmingham, Alabama. P Dhaliwal, MD, Brandon Regional Hospital, Brandon, Florida. SC Tiwari, MD, St. Dominic-Jackson Memorial Hospital, Jackson, Mississippi. KG Julian, MD, Hershey Medical Center, Hershey, Pennsylvania. Epidemiology and Surveillance Div, National Immunization Program; Div of Vector-Borne Infectious Diseases; Div of Global Migration and Quarantine, National Center for Infectious Diseases; EIS Officer, CDC.
This report documents two probable new cases of 17D-derived YEL-AVD and four probable new cases of 17D-derived YEL-AND in the United States. YEL-AND has long been recognized as a vaccine-associated adverse event, but incidence decreased substantially with implementation of the seed-lot standardization process in 1945. Since then, 27 cases of YEL-AND, including seven U.S. cases, have been reported worldwide (1,6). YEL-AVD was recently recognized; since 1996, 12 cases of YEL-AVD, including six U.S. cases, have been reported worldwide (1--4).
This report describes the first U.S. case of YEL-AVD in a person aged <50 years. Of the 12 cases reported worldwide, five were in persons aged <50 years. Similar to the YEL-AVD cases reported previously, onset of symptoms occurred 1--6 days after vaccination (1). Two of the four persons with YEL-AND became ill 13--23 days after vaccination.
YF is a flavivirus that causes a febrile illness in humans that can progress to hepatic and renal failure and hemorrhage caused by platelet and clotting abnormalities. In primates and mice, YF also can cause meningo-encephalitis (6). YEL is a live virus preparation containing 17D vaccine strain made by serial passage of wild type YF virus to attenuate neurotropic and viscerotropic properties while preserving immunogenicity (4). Sequencing evidence suggest that YEL-AVD and YEL-AND might represent an aberrant host response to 17D vaccine strain rather than a reversion of vaccine virus to wild type (1,3).
The cases of neurologic disease had evidence that 17D-derived YEL was the likely cause of illness. The four patients had onset of illness soon after YEL was administered and had high levels of YF-specific IgM antibody in CSF; no other causes of neurologic disease were identified. However, viral isolation of YEL-associated virus in these patients was either negative or not performed because of inadequate samples. The presence of IgM antibody in CSF might be caused by serum antibody from recent vaccination crossing an inflamed blood-brain barrier; however, this is unlikely because of the large size of IgM. The two patients with visceral involvement also had illness associated temporally with YEL, had clinical features similar to other reported cases of YEL-AVD (1--3), and had extensive diagnostic testing, excluding other infectious and noninfectious etiologies. However, tissue samples were not available for testing because both patients survived despite multiple organ system failure.
Enhanced surveillance was useful in identifying additional suspect cases of YEL-AVD and YEL-AND. These findings indicate the need for continued enhanced surveillance, timely clinical assessment, and a refined risk estimate for severe adverse events following receipt of YEL. However, enhanced VAERS surveillance efforts alone might not detect all serious adverse events after receipt of YEL (7).
Clinicians are encouraged to report promptly to VAERS any patients with symptoms suggestive of viscerotropic or neurotropic illness or any patients with fever of >101.3º F (>38.5º C) for >24 hours and illness onset <30 days following receipt of YEL. VAERS report forms are available online at http://www.vaers.org or by telephone, 800-822-7967. Completed forms can be submitted online; by fax, 877-721-0366; or by mail, P.O. Box 1100, Rockville, MD 20849-1100. Supplemental clinical information and information about the availability of clinical, autopsy, or residual vaccine specimens may be requested. CDC will conduct virologic and immunohistochemical studies of these specimens. Additional information is available from CDC at http://www.cdc.gov/ncidod/dvbid/yellowfever/index.htm and http://www.cdc.gov/travel and by telephone, 970-221-6400 and 404-498-1600.
Because of the potential severity of YF infection, YF vaccination is recommended for persons aged >9 months traveling to countries where YF is endemic or epidemic. YF has caused recent deaths in unvaccinated U.S. and European travelers to endemic areas of sub-Saharan Africa and tropical South America (8--10). To mitigate the risk for YEL-associated disease, health-care providers should provide YEL only to persons planning to travel to areas reporting ongoing YF activity or with a history of endemic transmission.


| Use of trade names and commercial sources is for identification
only and does not imply endorsement by the U.S. Department of Health and
Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
|
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
Page converted: 11/7/2002