Vaccines 97, Brown & al ed.,Cold Spring Harbor Laboratory Press, New York, 1997
A New Potential Threat in Antigen and Antibody Products: Nanobacteria
Neva Ciftcioglu, Ilpo Kuronen, Kari Akerman, Erkki Hiltunen, Jukka Laukkanen
and E. Olavi Kajander
Department of Biochemistry and Biotechnology, University of Kuopio, FIN-70211
Kuopio, Finland.
Several vaccines are currently being produced by using cultured mammalian
cells. Microbiological sterility of such vaccines is of great importance since
several examples indicate potential safety hazards in vaccines contaminated with
unknown organisms. Fetal bovine serum (FBS) used as a supplement in cell culture
is a known safety risk (Hodgson, 1995). Obviously, not all of the risk factors
of FBS are yet known and thus cannot be controlled. It is commonly known that
only about 10% of FBS batches support cell cloning well (Liddel and Cryer, 1991)
but the reasons for this have remained unclear. As with many other cell
culturers, we faced a problem about 10 years ago of poorly thrivingo cells not
attributable to any known contaminant. In this report, we describe the discovery
of a new bacterium from mammalian blood and blood products, tentatively named as
Nanobacterium sanguineum gen. et sp. nov., and show that this agent is
common and harmful.
DISCUSSION
Culture and Diagnosis of Nanobacteria
The discovery of Nanobacteria came about because we had a problem with cell
cultures namely vacuolized cells (Fig. 1A) and poorly thriving cultures without
any contaminant detectable by standard methods. Transmission electron microscopy
(TEM) made from these poorly thriving cell cultures indicated the presence of
internalized procaryotic organisms (Fig. 1B). That their source was the
commercial "sterile" FBS was proven by gamma-irradiating all the culture
components (Table 1). This experiment also indicated that sterile culture media
for detection of new organisms can be made by using gamma-irradiated serum as a
supplement. The new organisms passed through 100 nm (but not 50 nm) filters and
were called nanobacteria, since no other bacteria are known that can pass
through filters with such small pores. This ability to pass through such
small-pore filters was most remarkable since they were shown to have a cell wall
and yet were able to surpass the filterablity of cell-wall-less bacteria. They
were unculturable in microbiological media but could be cultured under cell
culture conditions (with or without mammalian cells, CO2 5-10%). These minute
generally coccoid organisms had a diameter of 200 to 300 nm in serum, and their
size increased during the culture due to the production of a very thick cell
envelope (Fig. 1C, D). The thick and calcified envelope made them visible even
by light microscopy. The doubling time of nanobacteria was 1-5 days (Fig. 2).
Their multiplication could be detected by specific ELISA, optical density,
microscopic counting, SDS-PAGE or methionine and uridine incorporation, and the
multiplication could be prevented with high doses of aminoglycoside antibiotics,
EDTA, cytosine arabinoside and gamma-irradiation. Considerable evidence
suggested the presence of nontraditional DNA. 16S rRNA gene sequence results
(data will be published elsewhere) placed them into the alpha-2 subgroup of
Proteobacteria which includes Brucella(which are also pathogens of
mammalians with preference to the fetus) and Bartonella.
Nanobacteria were isolated from more than 80% of commercial FBS and newborn
bovine sera and are the most common contaminant present in cell cultures. In
addition, we isolated nanobacteria from the blood of 4% of medical students at
our university. Positive identification of nanobacteria involved growth in cell
culture medium with typical growth rate and optical properties, specific
stainability with Hoechst 33258 using the high dye concentration and positive
immunoassay results with immunofluorescence and/or ELISA using monoclonal anti-nanobacteria
antibodies.Cytotoxicity of Nanobacteria
Nanobacteria are cytopathic in cell cultures and invade mammalian cells in a
distinctive manner: They trigger cells that are not normally phagocytic to
engulf them. These novel organisms are one of the causes for cell vacuolization,
poor thriving and unexpected cell lysis, problems often encountered in mammalian
cell culture. Several mammalian fibroblast lines were cultured in MEM medium as
described previously (Kajander et al., 1990), and were infected with
nanobacteria. Electron microscopy and FITC staining with specific monoclonal
antibodies indicated that nanobacteria were bound on the surface of the
fibroblasts (Fig. 1E-G). We concluded that they were internalized either by
receptor-mediated endocytosis or by a closely related pathway. After the
internalization, fibroblasts showed apoptotic abnormalities and died if
subjected to a high dose (>100 nanobacteria/cell).Different Growth Phases of
Nanobacteria
Washed nanobacteria added to serum-free medium grew very slowly as evidenced by
increase in their numbers and protein level and were firmly attached to the
culture plates. These cultures progressed to large multicellular formations
covered by layers of a firm protective material several micrometers thick (Fig.
1H). After addition of sterile serum, the layer disappeared, with typical small
coccoid nanobacteria later appearing in the same cultures with the mobile,
larger D-shaped ones (Fig. 1I). Specific monoclonal antibodies indicated the
presence of the same antigenic sites in both D-shaped and coccoid nanobacteria,
and their 16S rRNA gene sequences were 98% identical.How can Cell Culture be
Possible with Nanobacteria-contaminated Fetal Bovine Serum?
Although more than 80% of cell culture serum batches are contaminated with
nanobacteria, many cell culturers have not faced this problem with their cell
cultures. We have experienced a major problem with nanobacteria in cell culture
only when they are present at high concentrations relative to cells. This can
occure typically in cell cloning and in long-term experiments where mammalian
cells do not multiply. Internalization of numerous nanobacteria by a cell
results in cytotoxicity. Importantly, most cell lines multiply faster than
nanobacteria. Thus, cytotoxic concentrations may be avoided.Why is
Nanobacteria a Potential Threat?
Nanobacteria can cause a chronic infection in laboratory animals and in humans.
The agent could be isolated from blood of one peron for 5 years despite the
presence of antibody. When nanobacteria were injected into rabbits, the agent
was initially isolated from urine and then from cerebrospinal fluid after one
year. Nanobacteria multiply very slowly and, if pathogenic in humans, might
cause slow chronic autoimmune-like disorders (compare with leprosy or
brucellosis). Sofar, there are no chronic bacteraemia known that would not be
harmful. Thus, the posibility that nanobacteria may be present in vaccines made
with cell culture, or in gammaglobulin or other antibody preparations, must be
controlled.SUMMARY AND CONCLUSIONS
Nanobacteria are novel microorganisms that are not detectable with present
sterility testing methods, but they are detectable with new culture and
immunomethods. They are commonly present in bovine and blood products and thus
in cell cultures and antigens, including vaccines derived therefrom, and may be
present in antibody and gammaglobulin products. Nanobacteria are a potential
risk because of their cytotoxic properties and ability to infect fetuses, and
thus their pathogenicity should be scrutinized.
ACKNOWLEDGMENTS
We thank E. Tahvanainen, H. Martikainen, A. Pelttari and T. Ojanen for their
valuable help in microbiological, microscopic, and chemical techniques. P.
Mäenpää, T. Eloranta, J. Kärjä and O. Lindqvist contributed valuable ideas in
discussions. The work was supported by the Academy of Finland, University of
Kuopio, Finland Technology Center, Juho Vainio Foundation and Savo High
Technology Foundation.REFERENCES
- Hodgson, J. 1995. To treat or not to treat: That is the question for
serum. BioTechnology 13: 333.
- Kajander, E. O., R. J. Harvima, L. Kauppinen, K.K. Akerman, H.
Martikainen, R. L. Pajula, and S. O. Kärenlampi. 1990. Effects of
selenomethionine on cell growth and on S-adenosylmethionine metabolism in
cultured malignant cells. Biochem. J. 267: 767.
- Liddel, J. E., and A. Cryer. 1991. in A practical guide to monoclonal
antibodies, p. 25. Wiley, New York.
 Figure 1. Ultrastructure
of nanobacteria and their interaction with fibroblasts. Figure 1. Ultrastructure
of nanobacteria and their interaction with fibroblasts.
(A) Perinuclear vacuolization of an infected 3T6 cell under
phase-contrast microscope;
(B) TEM image of a nanobacterium engulfed by a BHK cell;
(C) cultured coccoid nanobacteria (bars 200 nm).
(D) SEM image of nanobacteria attached to culture vessel;
(E) nanobacteria attached to a fibroblast surface (arrow shows the
surface of the fibroblast; bars 1 µm).
(F) Indirect immunofluorescence staining of cultured healthy 3T6 cells
with a monoclonal antibody (8/0) against nanobacteria;
(G) 3T6 cells inoculated with nanobacteria;
(H) TEM of a nanobacterial population in a serum-free culture (arrow
shows a D-shaped nanobacterium in this population);
(I) D-shaped nanobacteria after culture in serum-containing medium (bars
1 µm). |
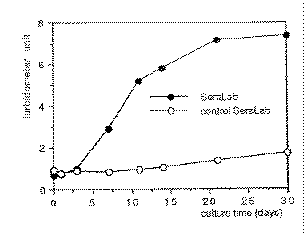 Figure 2. Growth-curve
of nanobacteria. As a control, gamma-irradiated FBS was used. At each
time point, samples from triplicate incubations were taken, frozen and
analyzed by turbidometer at the end of the experiment. Turbidometer
units are means of three measurements from 1/6 dilutions of cultures. Figure 2. Growth-curve
of nanobacteria. As a control, gamma-irradiated FBS was used. At each
time point, samples from triplicate incubations were taken, frozen and
analyzed by turbidometer at the end of the experiment. Turbidometer
units are means of three measurements from 1/6 dilutions of cultures.
|
Table 1. The Effect of 60Co Gamma-Irradiation of Culture Components on
Multiplication of Nanobacteria
| Culture |
Multiplication |
FBS
RPMI |
+ |
FBS
*RPMI |
+ |
*FBS
RPMI |
- |
*FBS
RPMI
nanobacteria or FBS |
+ |
*FBS
RPMI
*nanobacteria or * FBS |
- |
The material marked with asterisk (*) received a sterilization dose of 3
megarads during 16 h at room temperature. Cultures were established using 10 %
serum and nanobacterial counts were followed for 4 weeks.
Back Takaisin
