Autism. The dangers of using aborted fetal cell lines for vaccine manufacture
The dangers of using aborted fetal cell lines for vaccine manufacture have been debated by the FDA for over 50 years, and yet they have not done sufficient safety studies. The active component of a vaccine is a virus. Viruses are too large to manufacture in test tubes. Therefore, vaccine manufacturers exploit the natural method of producing virus– they inoculate cells and the cells produce the virus for them. Each vial of vaccine contains contaminants from the cells used to make the virus. When we use animal cells to make viruses, the residual material is not human and so we mount an immune response to it and eliminate it. However, in the case of vaccines produced using aborted human fetal cell lines, we have the dangers of triggering an autoimmune response and insertion of the contaminating DNA to disrupt the child’s own genes.
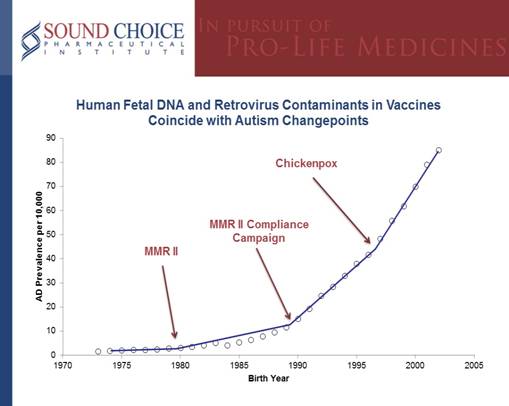
In the US, autism has spiked up in 3 distinct years, called changepoints. The first changepoint occurred in 1981, the second in 19881, and the third in 1996. These spikes coincide with the introduction of vaccines that are produced in aborted fetal cells. In 1979, aborted fetal cell produced MMR II was approved in the US. Compliance campaigns brought MMR II use up from as low as 49% for children born before 1987 to over 82% for children born in 1989 and later. A second dose of MMR II was also introduced to the vaccination schedule for children born in 1988 and later. The third changepoint corresponds to the approval of aborted fetal cell produced Varivax (chickenpox) in 1995 (See figure below).
The scientific community has well documented the biological processes that make human fetal contaminants in our vaccines so potentially dangerous. Sound Choice is doing the studies to demonstrate the actual dangers with each vaccine.
1Also published by the EPA in March 2010.
Further Information: