Institute for Vaccine Safety
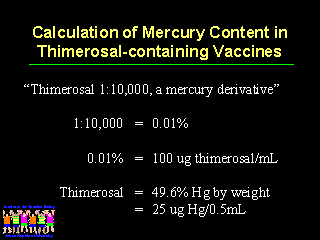
The quantity of mercury in each vaccine should be listed on the package label. Currently, the package label lists a concentration either as a dilution (ie, 1:10,000) or as a percent (ie, 0.01%).
As Leslie Ball from the Food and Drug Administration outlined in an earlier presentation, one must go through a two or three step calculation processes and know the molecular weight of each of the components of thimerosal in order to know that it is 49.6% mercury by weight, in order to determine the mercury content in each dose of vaccine. Since mercury is the biologically active component in thimerosal, the amount should be listed on the package label.

There are numerous factors associated with mercury toxicity, as noted on the slide. All children are not created equal with regard to their risk from exposure to mercury. We cannot control many of these factors, but we can have some control over the age and weight of children at the time they are exposed to mercury in vaccines
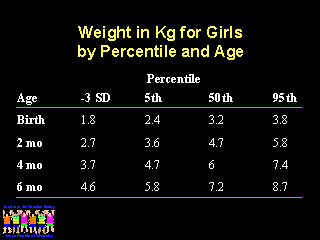
The smallest newborns can be less than half the weight of the largest newborns and this difference can continue until approximately 6 months of age. Guidelines should prevent potential harm from mercury exposure in this most vulnerable population, as pointed out by Kathryn R. Mahaffey, PhD, from the EPA. "If we take the weights of these children, and assume that they are receiving thimerosal-containing vaccines for each of the recommended vaccines by age, the following figure shows the dose of mercury in micrograms per kilogram that they would receive on the day vaccine is administered."
|
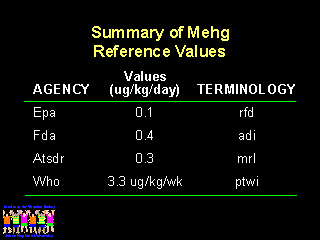
Which reference guideline for methyl mercury exposure should be used to evaluate ethyl mercury exposures from vaccines? WHO is the only organization that put a time period for exposure of more than one day, by giving a Provisional Weekly Tolerable Intake (PWTI). As noted by John Clements from WHO and Dr. Teely from the European Union, the WHO PWTI for pregnant women, nursing women, and infants would be one-fifth the PWTI for the general population. This makes the WHO guideline approximately the same as the EPA guideline. The Public Health Service has chosen the ATSDR guideline, which were based largely on the Seychelles data and allow for more liberal exposures.
Does that mean that we should ignore the data from the more recently generated studies in the Faroe Islands?
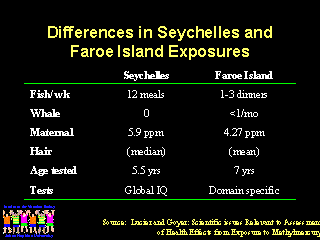
Dr. Lucier has summarized several possible explanations for the differences in the results of studies of children exposed in these two studies. In the Faroe Islands, there was evidence of harmful effects from maternal mercury exposures that were thought to be safe based on the Seychelles studies. In the Faroe Islands, exposure to mercury was primarily from intermittent bolus doses associated with consumption of pilot whales. Also, children in the Faroe Islands were examined at an older age than children in the Seychelles and more precise domain specific neurologic testing was performed. During the next two years, further follow-up data will become available from the Seychelles Islands using similar domain specific testing.
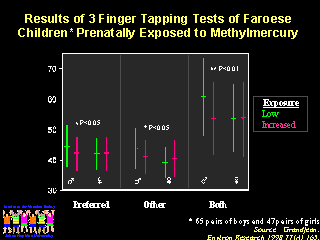
Testing in the Faroe Islands follow-up study required very sophisticated testing to detect very mild, subtle neurologic defects that would not be evident on routine examinations. The results provided many interesting observations, including the fact that the minor defects were noted primarily in boys. The biologic explanation for increased susceptibility to mercury of the male fetal brain has not been determined. There are other genetic factors associated with susceptibility to mercury toxicity which we may understand some day.
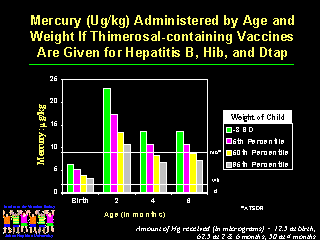
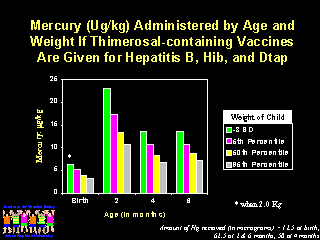
This figure shows the ATSDR guidelines selected by the Public Health Service superimposed on the daily exposures to ethyl mercury for children who receive all thimerosal-containing vaccines. At 2 months of age children of all weight categories receive more than 30 times the recommended daily maximum exposure and children of the smallest weight category receive almost three months worth of daily exposures on a single day.
If the EPA or WHO guidelines are used, the smallest children receive approximately 8 months of daily exposures in a single day. None of the guidelines provide us with the safety margin for these exposures or how single large exposures should be counted. Physicians administering vaccines and parents must make a decision at the time of immunization as to the safety of the dose of mercury delivered.
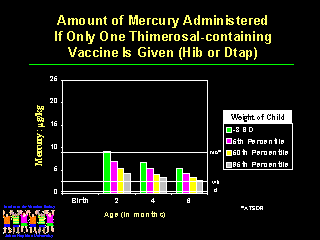
We need to tell physicians and parents about the options available for reducing mercury exposures. If only a single thimerosal-containing vaccine is administered such as Hib or DTaP at two, four and six months of age, then only the very smallest infants receive more than the total monthly mercury exposure allowed under the ATSDR guidelines. |
Slide 17 of 26 |
Slide Notes: Administering only hepatitis B vaccine gives one-half the mercury exposure and most children receive less than the weekly allowable dose after two months of age. |
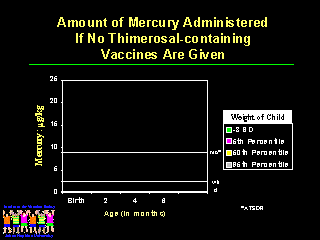
And it is possible to administer all of the recommended vaccines without using any thimerosal-containing vaccines.
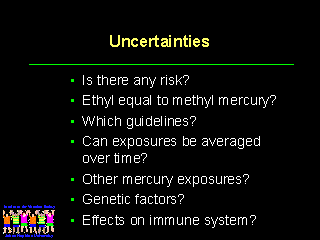
There are many uncertainties that have been discussed with regard to the mercury that children receive from vaccines. We need to share these uncertainties with physicians and the general public by providing more information and more specific guidelines and options.
We have not talked about the possible effects of mercury on the immune system. There are several centers doing immunologic studies on the effects of mercury on the immune system. We can expect many new publications on this subject during the next two years.
One thing that has not been highlighted enough is the fact that the mercury exposures from vaccines are in addition to background exposures to methylmercury from fish consumption.
This chart is from the EPA report to congress shows ranges of fish consumption in different populations within the United States. Most groups have low to moderate fish exposure, but there is great variability and there are numerous populations with very high levels of fish consumption in this country.
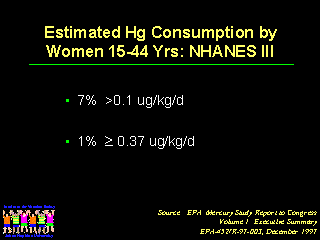
As noted by Kathryn R. Mahaffey, PhD, the EPA estimates that seven percent of women of childbearing age consume sufficient fish to have a mercury exposure of about 0.1 micrograms per kilogram per day, the upper level that EPA considers to be safe. The EPA also notes that about one percent of women of childbearing age are consuming 0.37 micrograms or more per kilogram of mercury per day, which is more than the ATSDR reference value.
Thus, some children are being born with mercury from their mother’s exposures that are above the guidelines provided by EPA, ATSDR, and WHO. Although safety factors have been built into these guidelines, we do not know what the effects of additional mercury will be on the developing brain.
Throughout the country there are warnings about mercury exposure from fish directed to pregnant women, nursing women, and in some cases for children as noted in this poster from Maine. Local populations, especially Native Americans, often ignore these guidelines.

Thimerosal is not a perfect preservative, as evidenced by numerous clusters of disease caused by DTP vials that had become contaminated with the group A streptococcus
Manufacturers should be encouraged to use unit dosing for vaccine delivery whenever possible. Not only does this preclude the need for preservatives such as thimerosal, but this can eliminate errors from improper reconstitution or mixing of vaccines. Although these errors occur infrequently, they can be harmful. There are drawbacks to unit dosing, including increased refrigerator space requirements, but newer developments may reduce space requirements. There are also increased costs associated with unit dose delivery, which will need to be addressed.
To maintain public confidence in vaccines, we should put the mercury content in the label. We also need to revise vaccine information statements to include specific information about mercury exposures. These forms are our primary tools for communicating potential risks to recipients of vaccines and parents.
We also need to provide physicians with more precise guidelines regarding maximum allowable exposures on a given day. Should we have separate specific recommendations for the highest risk populations? This has never been very practical, and I believe we need to have guidelines that are applicable to all of the population, including the most vulnerable.
Also, vaccines with little or no thimerosal should be preferred for use in infants as has been recommended by the European Union.
The last point is that we need to have good science used for decision making in the review of alternatives to thimerosal and the effects on the final product from reducing or removing thimerosal from vaccines. We expect scientists at the Food and Drug Administration to help manufacturers address these problems. Instead of increasing the budget to address increasing numbers of safety concerns in the past five years, the budget for research at CBER (Center for Biologics and Related Products) has decreased to one third of what it was in 1994. This trend must be reversed in order to maintain the tradition of good science at the FDA and protect children against disease with the safest possible vaccines.