Dec 2012 http://www.ageofautism.com
The World Health Organization’s policy on thimerosal and vaccines was confirmed in June 2002 on the basis of known to be false and flawed data in a British study sanctioned by the US Centers for Disease Control, subsequently included in the notorious Institute of Medicine review of Thimerosal and MMR, and only finally published in PEDIATRICS, the Journal of the Academy of American Pediatrics in September 2004. But it was known at the CDC before work on the paper began in 2001 that the data was unsuitable for reviewing the WHO program. It is essential to re-examine this matter in the light of the forthcoming UN decision over whether mercury should be retained in vaccines.
In 2001 the UK’s Sunday Times newspaper was taking an interest in the mercury content of vaccines, two years after the issue came to public light in the US. Against this background it reported on 17 June that the WHO would be launching an investigation into the safety of thimerosal (or thiomersal as it is called in the UK) led by epidemiologist Elizabeth Miller, head of immunization at the UK’s Public Health Laboratory Service (which was subsequently absorbed into the allegedly independent Health Protection Agency). The report stated:
“She will analyze records of 500 GP practices to check for a link between the use of vaccines that contain the preservative thiomersal — which is almost 50 percent mercury — and a range of neuro-developmental disorders which include autism.”
Responding to the newspaper all of a month later in what seems be a masterpiece of bureaucratic spin Miller wrote:
“Your articles, Autism linked to mercury vaccine' (May 27) and 'Inquiry launched into vaccine ‘link’ with autism' (June 17) implied that there has been increasing use of thiomersal-containing vaccines in the U.K. since 1988. In fact, the thiomersal content of vaccines given in the routine vaccination program has not increased over the past decade. The only vaccines for children used in the routine program that contain thiomersal are DPT (diphtheria, tetanus, pertussis ) and DT. Because of theoretical concerns that the small amount of mercury in thiomersal could be harmful, both European and United Kingdom regulators have recommended that manufacturers phase out its use wherever possible as a precaution.
“As a further precautionary measure, the Public Health Laboratory Service, on behalf of the World Health Organization, will be undertaking research into any negative effects of thiomersal-containing vaccines in the near future…”
On scrutiny this is less than re-assuring. In the first place Miller lurches casually from “since 1988” to “the past decade”, which is actually “since 1991” (thus evading the accelerated DPT schedule introduced in 1990) and she is only talking about the routine programme, in a period when many parents split the vaccines, unwittingly exposing their children to even more mercury. She states that the only vaccines that contain thimerosal are DPT and DT but that is only in the present, rather than over the previous 13 years. She inaccurately and prejudicially states that the amount of mercury in thimerosal is “small” (actually, as already stated by the Sunday Times, 50% by weight) and the concern theoretical. None of which boded well for a bias free investigation.
“The information given to me by the licensing authority is that the whole cell DTP/Hib vaccine we currently use contains 50 micrograms thiomersal per dose so that our children if on schedule would have 75 micrograms of ethyl Hg by 4 months of age. They originally told me that the whole cell DTP we used on its own from 1990 (when we adopted our accelerated schedule) up to 1992/3 contained 100 micrograms thiomersal, so exposure to ethyl Hg would have been 150 micrograms by 4 months. We then started using combined DTP/Hib vaccines for which the thiomersal content apparently was 50ug/dose. The authority is now saying that they have made a mistake and the vaccine we used up to 1992/3 only contained 50 micrograms thiomersal/dose. If this is true, then do we have sufficient exposure to ethyl Hg by 4-6 months of age to pick up an effect? Do I have to give my GPRD grant money from WHO back?”
Though it is disturbing that the head of the UK immunization laboratory service had to go to the licensing authority for information about the content of the vaccine it only half resolved her understanding and got her into further difficulty. What she has discovered was that there was insufficient mercury in the UK program to make a comparison with the WHO program, and she might have to give the money back (as we shall see this problem seems to have been eventually resolved by making a false statement in the study), but it also shows the British authorities were continuing to muddle the weight for ethyl mercury in thimerosal with the weight for inorganic mercury, a confusion which was still manifest in parts of the paper when it was published more than 3 year later.
The day before Chen received Miller's email he had received a sceptical comment on the project from his CDC colleague Thomas Verstraeten:
“I think two issues are important in assessing the potential strength of the GPRD study:
“1. Maximum exposure and 2. Unbiased controls.
“The maximum exposure is indeed relatively low if that was the only (Thimerosal) containing vaccine used. My estimate is that you need at least >50 by 3 months or >100 by 6 months to see an effect if there is one which you can barely make (50 at 2 [he means 3] mo and 75 at 4 mo in the UK).
“The quality of the comparison group is maybe even more important if you consider all the criticism we have received of comparing high T ([thimerosal] exposure to no or low T exposure. I am not sure if the GPRD [General Practitioners' Research Database] is that reliable that you can be sure that low exposure is really low exposure and not underascertainment in the database.
“I hate to say this, but given these concerns, it may not be worth doing this after all. On the other hand, maybe the grant can be given to Harald in Sweden to do his follow-up of the DTaP trial kids….”

Leaving aside momentarily the issue of the quality of the database, we
need to focus on the fact that the CDC were apparently in control of
whether the study went ahead (and as we see it was only more than two
weeks after this that Miller was free to announce that it would in a
letter published in the Sunday Times). But when, more than three years
later, the study was published - having been presented to both the WHO
and the IOM in the interim - it contained what looks like a blatantly
false statement about a matter which was considered in detail by the CDC
even before it started:
“Because the United Kingdom changed to an accelerated 2/3/4 month DTP immunization schedule in 1990 (replacing the former 3/5/10 month schedule) and because vaccinations are generally given on time in the United Kingdom, a substantial proportion of children in the GPRD cohort will have had a cumulative Hg exposure of 150 μg of thimerosal (75 μg of Hg) by 4 months of age. This level of Hg exposure, although lower than the maximum of 187.5 μg received in the United States by 6 months of age, is similar to the level received by ∼3 to 4 months of age in the United States. It is also the same as the amount of thimerosal used by developing countries that follow the expanded immunization schedule."
To be clear the WHO schedule was nothing like the UK “routine”
schedule and was even steeper in mercury exposure than the US,
administering the same 187.5 micrograms not in the first six months but
in the first three (to smaller, less mature and already much more
environmentally challenged infants). This information is confirmed in a
UK licensing authority document (which continues to confuse Hg with EtHg):

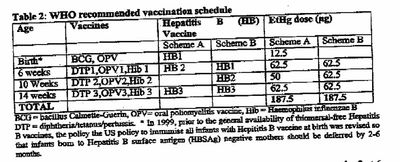
in which the UK exposure by 3 months is just 26.7% of the WHO program
and not remotely “the same” as stated.
This is not the only problem with the study and Verstraeten’s concerns about the quality of the database seem well justified. For instance, the authors only manage to identify an incidence of autism of approximately 1 in 1000 when a figure of 1 in 100 had been identified in a National Statistics children’s survey. Indeed, most of the actual autism cases must exist unidentified in the non-autistic group.
Several of the authors (Miller, Taylor, Andrews, Stowe) also took part in other studies (Lancet and Archives of Disease in Childhood) examining the rate of autism in relation to MMR in North London yet they failed to note the rise in autism associated with the introduction the accelerated DPT schedule in 1990, and they also failed to note this factor as a confounder to their negative analysis of the relationship between the introduction of MMR and autism. Miller and Taylor would already have known this from the Taylor Lancet paper of 1999. Plainly, if they had done a time trend study on the accelerated DPT they could have detected an effect, and it would have made it harder to deny that there was one with MMR as well.
The authors failed to disclose commercial conflicts as Mark and David Geier wrote in due course to PEDIATRICS:
“The authors of the Andrew[s] et al. study failed to disclose their significant conflicts of interests to the readership of Pediatrics: Elizabeth Miller disclosed in her 2001 publication…and in 2002 to the Committee on the Safety of Medicines previously disclosed that she has received funding to study vaccines from Aventis Pasteur, Wyeth Vaccines, SmithKline Beecham, Baxter Health Care, North American Vaccine, Wyeth- Lederle Vaccine, and Chiron Biocine; and Nick Andrews, Julia Stowe, and Brent Taylor all disclosed in 2001 that they received funding to study vaccines from Wyeth Vaccines and SmithKline Beecham… These companies all are or were makers of thimerosal-containing vaccines.”
Additionally, Prof Taylor failed to disclose that he sat on the UK’s Joint Committee on Vaccination and Immunisation and therefore had direct collective responsibility for the policy .
The study had numerous exclusions, for instance “Children were also excluded when they received either hepatitis B or influenza vaccination in the first 6 months of life because such children are likely to be an atypical subgroup”, but they would also have had greater exposure to mercury which they do not mention.
With all this the authors failed to disguise the increased occurrence of tics.
In line with careful news management the study was scheduled to be published simultaneously with the phasing out of the use of thimerosal containing pediatric vaccines in the United Kingdom, which, however, the British government continued to license for use abroad.
Tell Washington USA Should Not Export Mercury to Global Children
This article is largely based on research in two earlier articles:
http://www.jabs.org.uk/john-stone-writes.html
http://www.vaclib.org/sites/vap/pediatrics-validated-erroneous-mercury-data-20070327.htm