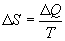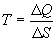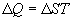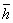2.1.1
Introduction:
In part 1 one we covered the scientific and societal controversy
surrounding Maxwell's daemon, and briefly touched on two devices
that exhibit anti-entropic behavior. In part 2 we shall take an
in-depth look at the mechanism responsible for anti-entropic
behavior in the laser. This mechanism is called a population
inversion, and until the mid twentieth century, was considered
little more than a quaint mathematical curiosity, with no basis in
physical reality. In order to lay a proper foundation for
discussion of inverted populations, sections 2.1.2 and 2.1.3 are
included as a short review of classical thermodynamics.
2.1.2
Degrees of Freedom:
At it's most basic level, the science of thermodynamics is a
statistical study of vibration and movement in populations of atoms
or molecules. In order to numerically quantify a population, it is
necessary to understand how many different ways or modes of
vibration and/or movement are available to the population. For
instance, a population of molecules that have magnetic properties,
when under the influence of a magnetic field, may be constrained to
movement in a single direction, and yet in the absence of a magnetic
field, this same population may have nearly unlimited directions of
movement. In thermodynamics we use the term "degrees of freedom" to
describe the number of available modes of vibration and/or
directions of movement. Alternately, degrees of freedom can be
viewed as the set of locations available to a population of atoms or
molecules when it moves or vibrates. This set of available
locations is called the population's "phase space".
2.1.3:
Temperature, energy, and entropy:
The concept of temperature arose from a need to quantify the human
perception of hot and cold. However at the beginning of the 19th
century, no scientist was sure what temperature was really
measuring. It was well known that expending energy (doing work),
such as drilling a hole, or bending sheet metal caused the material
to get hot, so heat was related to energy in some manner. But what
was heat? Some believed it was a discrete substance, separate from
matter (they called it caloric), while others believed it was an
inherent property of matter, like mass or volume. In 1877, the
debate was settled by the mathematician Ludwig Boltzmann. He showed
that heat is a property of matter, directly related to the energy
stored in it's vibration and/or movement, AND related to the entropy
(disorder) of that vibration or movement. Unfortunately his
theories were met with extreme skepticism by the scientific
community of that era (Maxwell was an exception). Despondent, he
later committed suicide. Had he lived just a few more years,
Boltzmann would have seen his theories vindicated beyond his wildest
dreams. Scientific luminaries such as Albert Einstein and Max
Planck used Boltzmann's theories in their work. Work that has
shaped modern quantum physics as we know it today.
Boltzmann's controversial equation, Eq. 1 relates the entropy of an
atomic or molecular population to it's phase space (disorder). As a
side note, this equation was engraved on his tombstone...
[Eq. 1] S = k Log W
Where:
Form our perspective, the utility of Eq. 1 is that it defines
entropy (disorder) as the ratio of energy to temperature. Looked at
another way, given a population with a constant energy content, as
entropy declines, temperature rises. In other words, as the degrees
of freedom available to a population diminish, those few degrees of
freedom still available, MUST contain the entire energy content of
the population, and this causes the temperature of the population to
rise.
Thanks to the genius of Boltzmann, we can define the relationships
between temperature, energy, and entropy
One last observation is relevant before moving on. Eq. 2b implies
that for any population with a non-zero energy content, as entropy
approaches zero (one degree of freedom), the temperature of the
population approaches infinity.
2.1.4
Maximum disorder:
Is there an upper limit to disorder? The answer to this question
depends on the degrees of freedom available to a population of atoms
or molecules. For most populations, and in the most general sense
of the question, the answer is "no". This answer has a practical
consequence. It implies there is no upper limit to temperature,
since there will always be another dimension in phase space (degree
of freedom), to which we can add another increment of energy, and
thereby raise the temperature of the population.
However, if a population has a limited set of dimensions in phase
space (limited degrees of freedom), there is a definite upper limit
to disorder. Surprisingly, the limit will be reached when exactly
half of the dimensions in phase space are occupied by the
population. It is obvious that when a population of atoms or
molecules contain no energy, they do not vibrate or move, and
therefore all members of the population occupy a single point in
phase space (no disorder). Now consider the opposite condition.
I.E. Every member of the population is vibrating or moving in every
available degree of freedom (all dimensions of phase space are
occupied). Again, all members of the population are exactly alike,
and again there is no disorder. Therefore maximum disorder (and
entropy) is achieved when exactly 50% of the dimensions in phase
space are occupied. As a consequence of this remarkable situation
Eq. 2b implies the temperature of a population at maximum disorder
is infinite, and the temperature of any population where more than
50% of phase space is occupied is negative AND hotter than infinity.
Figure 1 shows the relationship between temperature and phase space
occupancy for a population with limited degrees of freedom.

Figure 1 - Temperature versus phase space occupancy
2.2.1
Quantum populations:
While classical physics allows populations of atoms or molecules to
have nearly unlimited modes of vibration and movement (with
corresponding degrees of freedom), quantum electrodynamics is a
different story altogether. Consider the electron orbits of a
Hydrogen atom. The allowed orbital values are discreet, and defined
by Eq. 3. Therefore electron orbits represent a population with
very limited degrees of freedom (dimensions in phase
As a consequence of Eq. 3, an atom will only absorb (or emit)
electromagnetic energy at those frequencies corresponding to the
difference in energy between allowed orbits. In a population of
Hydrogen atoms at room temperature, the vast majority of electrons
are in the orbit N = 1, and the effective temperature of the orbital
population is very close to zero. Now suppose we pass a controlled
electric current through the Hydrogen atoms, thereby raising the
majority of electrons into the orbit N = 2. We now have a
population with only 2 degrees of freedom (two dimensional phase
space) N = 1, and N = 2. Further, since the majority of electrons
(greater than 50%) are in the orbit N = 2, the population of
electron orbits is at a negative temperature (see figure 1 above),
and therefore hotter than infinity. In thermodynamics terms, this
is known as a population inversion, and under the rules of classical
thermodynamics, was considered a physical impossibility...
2.2.2
The Laser:
In 1960, based on the thermodynamic principal of population
inversion, Theodore Maiman invented the laser (U.S. Pat 3,353,115).
An intense source of light or electric current is used to excite
electron orbits into a population inversion. The only way these
electrons can cool down is to emit a beam of electromagnetic energy
(photons) at a single wavelength, equal to the energy difference
between electron orbits (2.2.1). Since the population of photons in
the laser beam is of one wavelength (monochromatic), they have
exactly one degree of freedom, and as shown in (2.1.3), for any
non-zero energy, when S = 0 (W = 1 in Eq. 1), T is infinite.
The laser uses the population inversion of electron orbits as a
"daemon-like, trap door" to convert low temperature energy into a
coherent beam of light, hot enough to burn a hole through the Sun
2.2.3
The second law:
The second law of thermodynamics states that:
"In any cyclic process, the entropy must either increase or remain
the same".
Consider a steam engine. Steam engines can not convert all of the
energy contained in the boiler to useful work. Some of the energy
must be dumped into a condenser. The reason for this prerequisite
is that extracting energy from a boiler lowers the entropy of the
boiler, however dumping a smaller quantity of energy into a
condenser (at a lower temperature) raises the entropy of the
condenser more than entropy was lowered by extracting the larger
quantity of energy from the boiler (2.1.3, Eq. 2a). In other words,
a condenser is needed to meet the requirements of the second law of
thermodynamics.
For steam engines, or any other engine that operates on the
principal of heat extraction, the second law of thermodynamics IS
absolute (much to the benefit of OPEC). If steam engines could
break the second law of thermodynamics, then a steam engine could
convert all of the energy from a boiler operating at room
temperature, without needing a lower temperature condenser to dump
waste energy.
2.2.4
Inverted populations:
Consider an engine extracting energy from a boiler containing an
inverted population of atoms or molecules. Since the act of energy
extraction, raises the entropy of the boiler (2.1.4, 2.2.1), this
engine DOES NOT require a condenser, and this engine will convert
ALL of the extracted energy into useful work. In other words, ANY
boiler operating on the right hand side of figure 1 (beyond 50%
phase space occupancy), is also operating beyond the point of
maximum entropy (disorder), and unlike a conventional boiler,
entropy increases as energy is extracted from an inverted population
boiler. Therefore no other step is required to meet the condition
imposed by the second law.
We have just uncovered a loop hole in the second law of
thermodynamics. This loop hole does NOT allow us to break the
second law of thermodynamics. Rather, the loop hole allows us to
neatly "side step" the consequences of the second law, as it's
traditionally understood to apply, with respect to heat driven
engines. In other words, an inverted population exhibits
anti-entropic behavior.
Or in more poetic terms, when operating on an inverted population,
the spirit of Maxwell's daemon LIVES!
2.3.1
Conclusions:
That inverted populations can be created (2.1.4, 2.2.1, 2.2.2).
That inverted populations exhibit anti-entropic behavior (2.2.4).
That inverted populations can be used to side step certain
consequences of the second law of thermodynamics as it applies to
heat driven engines (2.1.3, 2.1.4, 2.2.1, 2.2.2, 2.2.3, 2.2.4).
That when operating on an inverted population, the second law of
thermodynamics DOES NOT preclude the existence of daemon-like "trap
door" structures, as first envisioned by Maxwell (2.1.4, 2.2.1,
2.2.2, 2.2.4). In summary, some scientists will claim that while my
arguments are theoretically correct, they have no basis in physical
reality. I would invite these scientists to aim a high power laser
at their head, then carefully examine the "theoretical hole" in
their head.
End.
Magneto Thermodynamics, Part 2
|