Alan Cantwell, M.D Cancer Microbe
The Cancer Bacteria Conspiracy
Why Doctors Ignore The Bacterial Cause Of Cancer
© 2016 Alan R. Cantwell, M.D.
10-10-16
Cancer is caused by bacteria easily visible by use of the light microscope. This is my conclusion after 40 years of observing microbes in certain diseases of unknown origin, and in cancer tissue preparations prepared by pathologists. A century ago doctors were taught that bacteria do not cause cancer; and this belief continues to this day. Needless to say, I am sure most of my colleagues considered my search for bacteria as a futile waste of time.
When bacteria are found in cancerous tissue within the body (in vivo), they are generally regarded as “secondary invaders” or “opportunists” taking advantage of tissue weakened by cancer. Bacteria cultured from cancer tumors are often viewed as “laboratory contaminants” of no etiologic importance, or germs of the “normal flora.” On the other hand, billions of dollars have been spent trying to prove that viruses cause cancer.
Viruses are smaller than bacteria and cannot be seen with the light microscope. In my experience, bacteria are consistently visible in vivo in cancer, particularly when a special tissue stain is used, and if you know what to look for.
There has always been a small group of fringe scientists claiming that bacteria cause cancer. The most productive and vocal were four women (Virginia Livingston MD, microbiologist Eleanor Alexander-Jackson, PhD, cell cytologist Irene Corey Diller, PhD, and tuberculosis research icon Florence Seibert, PhD). During the 50s, 60s, and 70s, they confirmed each other’s work and reported cancer bacteria in various medical and scientific journals. Sadly, the women are now largely forgotten and their discoveries seldom mentioned in cancer circles.
With the establishment of the Human Microbiome Project by the NIH in 2008, there has been a flurry of renewed interest in microbes contained within the human body. Newly revised estimates indicate 40 trillion bacterial cells and 30 trillion human cells reside within the average-size man. Under conditions of health, these trillions of germs live in harmony with human cells. However, this “symbiosis” can become unbalanced with tissue injury or immune dysfunction. .
What is the ‘cancer germ”?
The microbe of cancer is unlike any germ I learned about in medical school or encountered in microbiology and pathology textbooks. It is essential to recognize that the cancer microbe in vivo is cell wall deficient (CWD). CWD bacteria (also known as L-forms) have lost a part or all of their cell wall. As a result, they are “pleomorphic,” meaning they can exist in more than one form in tissue (in vivo) and in laboratory culture (in vitro). (For details, Google key words ‘pleomorphic cancer bacteria’ and see ‘A history of cell wall deficient forms’ at http://bacteriality.com/2007/08/history.) CWD bacteria are resistant to antibiotics and often require special growth media for lab culture. The smaller forms in vivo do not stain well with the Gram stain, traditionally used to detect bacteria in tissue. They stain poorly (or not at all) with the routine hematoxylin-eosin tissue stain used universally by pathologists to diagnose disease pathology and cancer.
The microbe appears both within cells (intracellular) and outside the cell (extracellular), scattered about the tissue. With the highest magnification of the light microscope (1000 times) and with the oil immersion lens, the germ most commonly appears as the tiniest of barely visible round granular forms, or as larger round coccoid forms roughly the size of ordinary staphylococci. Less commonly, CWD bacteria appear as even larger round fungal and yeast-like round forms called “large bodies” or “globoids,” some attaining the remarkable size of red blood cells, or larger.
Microbiologists have demonstrated that the smallest forms of CWD bacteria are submicroscopic, filterable, and virus-like in size. For those unfamiliar with the various morphologic appearances of pleomorphic CWD bacteria, it is difficult to conceive of bacteria capable of such microbial variation.
Livingston and Alexander-Jackson discovered the acid-fast stain (used to detect the rod forms of tuberculosis bacilli by coloring them red) was useful to demonstrate the intermittently acid-fast cancer microbes in vivo and in vitro. The coccoid forms are usually not acid-fast (red-stained), but may stain them semi-acid-fast (purple). (For more on tissue staining and photos of the microbe, Google: acid-fast cancer bacteria.)
Bacteria cultured in vitro from cancer tumors may appear as ordinary staphylococci, streptococci and round and rod-shaped cocco-bacillary forms. However, due its intermittent acid-fastness and pleomorphism, the cancer microbe seems most closely related to the acid-fast mycobacteria, species of which cause TB, leprosy and dozens of other conditions.
Why is it important to identify bacteria in cancerous tissue?
The proof that bacteria cause cancer rests in consistently recognizing the microbe in vivo in pre-cancerous and cancerous tissue. I have observed the build-up of bacteria in sun-damaged precancerous skin lesions. This is evidence the bacteria are present before full-blown skin cancer forms; and proof they are not merely “secondary invaders” or “contaminants.”
Much of the continuing controversy in cancer bacteria research has concerned the exact nature of the microbe. Is there one causative specific species involved, or more than one type of bacteria? I consider this controversy a current distraction for this reason: It is unproductive to quibble about the exact nature of the microbe before there is any agreement as to whether an actual cancer microbe routinely exists in vivo in cancer in the first place.
How to recognize cancer bacteria in cancer tissue
The cancer microbe hides in plain site. Its origin is most likely from the trillions of bacteria already in the body. CWD bacteria also exist in the blood of all humans. Using newer molecular biology techniques, multiple species of bacteria have been detected in various body tissues and organs previously thought to be sterile (bacteria-free). Some scientists now suggest that body bacteria might play a role in cancer and other chronic diseases
In size and shape, the smallest forms of CWD bacteria in vivo can individually resemble various cellular “granules.” But, unlike non-microbial tissue granules, one can observe the increasing growth of microbial granules and coccoid forms into “large body” and “giant large body” forms of CDW bacteria. In the 1890s, these large forms were intensively studied by pathologist William Russell, who thought they represented “the parasite of can cer.” Pathologists recognize “Russell bodies” in vivo, but consider them non-microbial in nature.
Figures 1-5 (below) show examples of pleomorphic cancer bacteria in tissue (in vivo) and in lab culture (in vitro) in four forms of cancer: breast cancer, prostate cancer, Hodgkin’ lymphoma and AIDS-related Kaposi’s sarcoma. Figures 6-10 show similar forms in the bone marrow of an autopsied AIDS case; and in the lymph node of another AIDS case along with bacteria cultured from the blood shortly before death. Note how the size and shape of some of the cultured bacteria (staph, strep, corynebacteria) are “dead ringers” for some of the specially-stained bacterial forms (granules, coccoid forms and “large bodies”) seen in vivo in the cancerous and diseased tissue.
Undoubtedly, every pathologist has seen the in vivo forms presented here. I contend they are microbial CWD bacteria forms; pathologists consider them non-microbial. In this regard, Gerald J Domingue, an authority on CWD bacteria, writes in ‘Demystifying pleomorphic forms…Are they bacteria?’(available online): “These forms have been observed in stained tissue histopathologic specimens for many decades, most are ignored and generallly regarded as diagnostically insignificant staining artifacts or debris.” He hypothesizes that such objects may “represent vatious stages in the life cycle of stressed bacteria,”
Is there or is there not unrecognized bacteria in cancer tissue?
Surely it would be a no-brainer for cancer experts to determine, once and for all, if CWD bacteria are consistently found in vivo in cancer? Over the decades I have reported such bacteria in breast cancer, Hodgkin’s and non-Hodgkin’s lymphoma, classic and AIDS-related Kaposi’s sarcoma, AIDS-related immunoblastic sarcoma (B-cell lymphoma), mycosis fungoides (T cell lymphoma), and in certain non-cancerous chronic diseases, such as scleroderma, lupus, and sarcoidosis. These papers (posted on the PubMed.gov website) contain numerous references to dozens of cancer microbe researchers dating back to the nineteenth century.
Why are bacteria ignored in cancer?
Bacteria were discovered in the late 1670s, more than two centuries before doctors finally accepted them as disease-causing agents. It’s sobering for me to realize my grandparents were born in an era when doctors didn’t believe germs caused any human disease.
Bacteria still escape easy detection even in this modern age of medical science. In July 1976, an outbreak of a new and sometimes fatal pneumonia-like disease broke out among 222 Legionnaires and their families staying at a Philadelphia hotel. For months the cause was a complete mystery. The top medical scientists, physicians, pathologists, infectious disease experts, and microbiologists could not solve it. Tests for bacteria were all “negative.” Lab anmals were injected with the diseased tissue. Almost a year later, CDC microbioligst Joe McDade, using a special stain on the animal tissue, uncovered bacteria that were
the cause. Later, it was determined that this “new” microbe (Legionella pneumophila) was involved in previous small pneumonia outbreaks.
Until the 198Os, doctors believed bacteria could not thrive in the acid environment of the stomach. Stomach ulcers were often thought to be stress-related. Now we know that certain pleomorphic stomach bacteria (Helicobacter pylori) can cause ulcers that can lead to cancer. A Nobel Prize was awarded in 2005 to two Australians for proving this bacteria-ulcer-cancer connection. Historically, this microbe was first observed in 1939 by pathologist A. Stone Freedburg, but his research found little support and no confirmation; and he discontinued his research. .
The disinterest or unwillingness of the medical establishment to conduct further serious cancer microbe research is a major injustice, not only to the spirit of medical science, but to patients who have died of cancer, to those who have cancer, and to those who will develop the disease in the future.
What about the other causes of cancer?
Everyone knows that smoking can cause lung cancer, and radiation from the sun can promote skin cancer. So aren’t they the cause? There are many factors and carcinogens that increase the likelihood of cancer. However, the one thing all these factors have in common is they damage cells of the body. Eventually this leads to inflammation of the tissues. Inflammation is a precursor to cancer and is always accompanied by the build-up of bacteria.. Our trillions of body bacteria are neither friend nor foe; they simply respond to cellular damage by increasing in number and becoming “opportunists.”
Is there a cancer conspiracy?
Virginia Livingston, my mentor and great friend, was widely viewed as a quack doctor until the day she died. In her last years, she even evoked the wrath of Robert Gallo, the most famous medical doctor and scientist in the world for his co-discovery of the AIDS virus. Regarding her cancer microbe research and proposed therapies, he ranted to the Los Angeles Times (April 6, 1984), “What is going on in this country? This is insanity! She can have her theories and what can I say? I don’t know of anything to support it. I can’t see any basis and I don’t know what to say or what analogy to give you.”
Livingston’s current Wikipedia page presents a rather unflattering portrait of her life’s work, noting that the American Cancer Society categorically denies her bacteria theory. Nevertheless, there are citations and links to seven of her many important papers published in scientific journals, most co-authored by colleagues.
After more than 100 years, the belated recognition of a cancer germ would undoubtedly make the medical establishment look foolish. It would create havoc in cancer research, particularly cancer virus research. And highly priced cancer drugs and therapies would have to be re-evaluated for their effect (on non-effect) on bacteria in cancerous tissue.
Despite their microscopic size, the trillions of cancer bacteria within our bodies are the unrecognized elephant in the room in cancer research. They are the only carcinogenic and infectious factor never seriously considered as a major cause of cancer.
For a long time I have been labeled a “conspiracy theorist” in the Wikipedia and elsewhere on the internet. So, I may not be taken seriously when I state that there is indeed a conspiracy to suppress cancer microbe research and ignore bacteria in cancer. What do you think?

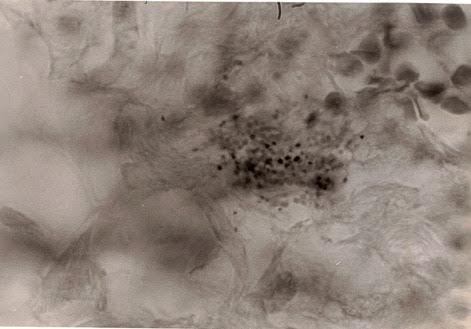
Figure 1. Tissue section of breast cancer showing numerous
extracellular coccoid forms in the tumor area.
The wrinkled large round forms at the upper right are red blood cells. Fite
(acid-fast) stain, magnification x1000, in oil.
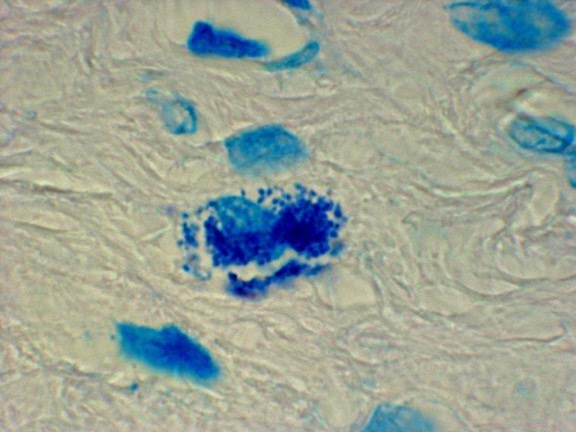
Figure 2. Tissue section of prostate cancer showing intracellular coccoid forms within the stroma. Fite stain, x1000, in oil.
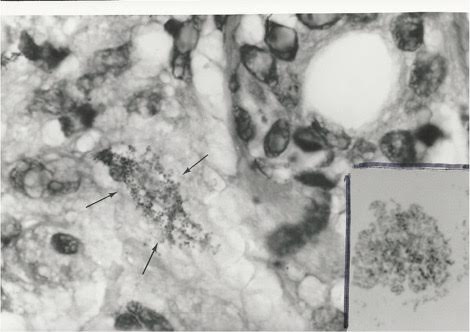
Figure 3. Tissue section of Hodgkin’s lymphoma of the skin
in an elderly man. Arrows point to tiny granular forms of CWD bacteria in the
dermis of the skin.
Fite stain, x1000, in oil. Insert shows cocco-bacillary coynebacteria-like
microbes cultured from the tumor. Compare the size and shape of the bacteria
found
in culture to the granular forms in vivo in the skin. Ziehl-Neelsen (acid-fast)
stain, x1000, in oil.

Figure 4. Tissue section of connective tissue in an
autopsied case of Hodgkin’s lymphoma occurring in a teen-age girl.
The two views show variably-sized intracellular and extracellular coccoid forms
exuding from the cell.
Some forms are similar in size and shape to common staphylococci. Fite stain,
x1000, in oil.
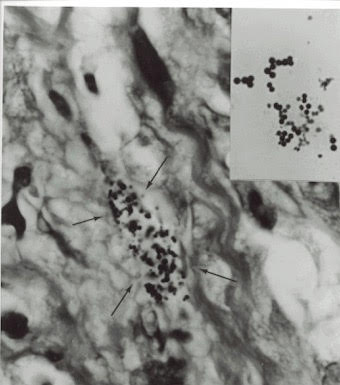
Figure 5. Tissue section of AIDS-related Kaposi’s sarcoma,
Arrows point to extracellular coccoid forms in the dermis portion of the skin.
Fite stain, x1000, in oil.
Insert shows Staphylococcus epidermidis cultured from the tumor. Gram stain,
x1000, in oil. Note the similar size and
shape of the bacteria cultured in vitro to the coccoid forms seen in vivo in the
tumor.
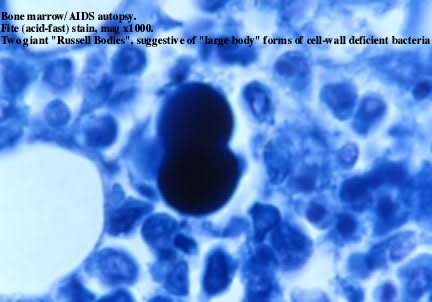
Figure 6. Tissue section of bone marrow in an autopsied
case of AIDS associated with Kaposi’s sarcoma. Shown is a “giant” budding “large
body”
form of CWD bacteria. This CWD form is much larger than a red blood cell. Fite
stain, x1000, in oil.
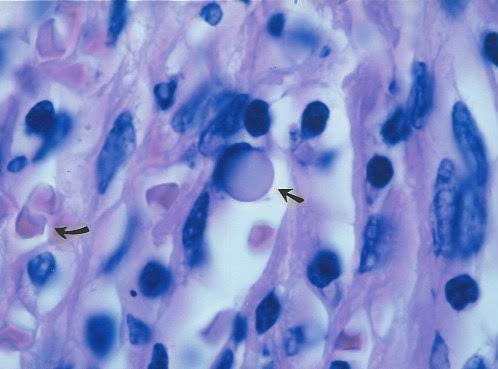
Figure 7. Tissue section of an enlarged lymph node
diagnosed as “reactive intrafollicular hyperplasia” in a fatal case of AIDS with
Kaposi’s sarcoma.
Arrow points to a “giant” transparent large body. Such forms are known to
pathologists, who generally consider them as non-microbial Russell bodies of
controversial origin.
Curved arrow points to two red blood cells. Staphylococcus epidermidis was
cultured from the node but was considered a contaminant, Hematoxylin eosin
atain, x1000, in oil.
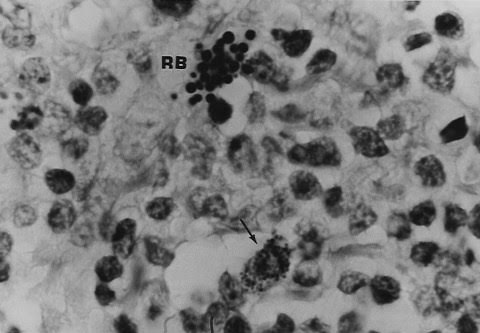
Figure 8. Tissue section of same lymph node shown in Figure
7. Arrow points to tiny intracellular granular and coccoid forms. Close by are
variably-sized “large bodies”
(also consistent with Russell bodies). Staphylococcus epidermidis was cultured
from the node but was considered a contaminant, Fite (acid-fast) stain, x1000,
in oil.
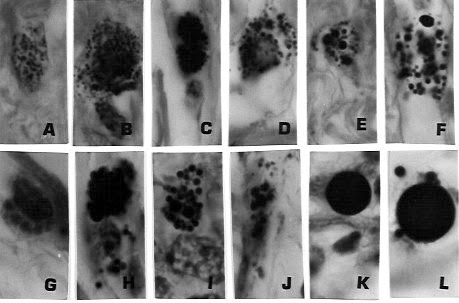
Figure 9. Tissue section of same lymph node as shown in
Figures 7 and 8. A through E shows the in vivo transition of tiny intracellular
and extracellular granules and
coccoid forms into progressively bigger “large bodies” (F thru J) and finally
into “giant” large bodies (K and L). Figures 9A-G, J,
stained with Giemsa; H stained with Fite; K.L stained with Gram stain. x1000, in
oil.
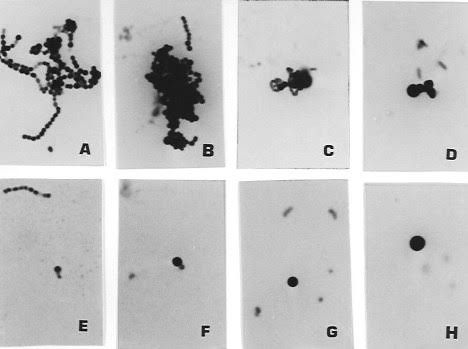
Figure 10. Pleomorphic forms of Streptococcus G cultured
from the blood one day before death of the AIDS patient whose tissue is shown in
Figures 7-9.
Figure 10A shows the classic chain of beaded coccal forms of Strep, Rare small
rod forms are sshown in 10D, Note the variably-sized round coccal forms in
10B-H; and compare in size
and shape with the coccoid forms, “large bodies” (Russell bodies) seen in
Figures 7-9. Ziehl-Neelsen (acid-fast) stain (A-E); Gram stain (B-D, F-H),
x1000, in oil.
[Alan Cantwell is a retired dermatologist. His books “The Cancer Microbe” and “Four Women Against Cancer” are available through Amazon.com]