ANIMAL RESEARCH T A K E S LIVES
- Humans and Animals BOTH Suffer
<< previous page | next page >>
contents | index
THALIDOMIDE
ARSL PAGE 7
ARSL 2nd Edition Page 9
On page 7 of ARSL it is claimed that: Animal research can not give any guarantee of absolute safety for any medicine, but that by testing medicines designed for human beings on a wide range of laboratory animals for several generations, the risks are reduced to very low levels. Also, that: The Thalidomide tragedy resulted from insufficient animal-testing.
Animal research can not give any guarantee of absolute safety for any medicine as is amply demonstrated by the increasing number of drugs withdrawn from the market due to their ill-effects. Further, it creates a state of uncertainty and confusion, and reliance upon it has inherent dangers of which we are bluntly reminded from time to time when medicines like Thalidomide instead of curing patients have the reverse effect.
ARSL's statements rest on the assumption that the principle of testing on animals, medicines designed for human beings, is authentic or bona fide. However more and more doctors are advising, some with extreme vehemence, that this principle is erroneous, incorrect, flawed. They argue vociferously and consistently that more tests on rats, mice, guinea-pigs, monkeys, cats and dogs are not merely useless and superfluous as extrapolations of information cannot be made to fit the human circumstance, but that they should be immediately abolished and a change to the principle of scientific anti-vivisectionism implemented. The Thalidomide tragedy arose from reliance on a faulty system which results, not as ARSL suggests in risks of "very low levels" but in risks of extremely high levels - enough to pale the tragedy of Thalidomide into insignificance as reliance on this faulty method causes increasing drug-failures promising future disasters of even greater magnitude.
WHY ANIMAL TESTING?
The end of the Second World War heralded an era in which factories involved in churning out chemicals for mass destruction of human beings switched to the development of wonder-drugs to cure their every ill. In the rubble of war-torn Germany in 1946 a factory employing 1,500 workers engaged in producing soap and detergents formed a subsidiary company called Chemie Grunenthal which began operations in an abandoned copper foundry near Hamburg. For several years it produced antibiotics, some for the American drug company Lederle with whom it had close links. Then, in the early 1950s came a series of failures as a number of its drugs produced severe adverse reactions including deaths. Though these were well reported and documented by several doctors Chemie Grunenthal ignored the serious risks involved with its dangerous preparations and moved into the profitable field of sedatives and hypnotics - which included Thalidomide.
The vital factor for the marketing of these modern-day medicaments was the investigation of how they react and interact with the human living organism. Though drugs produce different reactions in different animal species (and even between the same species), and though through the ages experiments on animals had been carried out for curiosity and were never intended to be used as confirmation of reactions experienced in human beings, this very inconclusiveness was the key factor in the choice of test method for it facilitated the marketing of hundreds of thousands of pills and potions for sickness, some of which never existed, much of which is self-inflicted and preventable, and an increasing amount of which is caused by previous drugs. The principle of animal testing however enabled the drug manufacturers to claim that the drug had undergone the usual safety tests. Thus animal tests were designed as legal protection for the company producing the medicine should this be required in a court of law, and not as protection for the patient. It is the solid-gold alibi for were conclusive methods of assessing medicines adopted, most would be exposed as worthless, if not dangerous, and consequently would never be marketed. For example:
The first Thalidomide prescribed by Chemie Grunenthal was produced at the beginning of November 1956 under the name of Grippex and prescribed for the treatment of respiratory infections. It was next marketed in West Germany on October 1 1957 under the name of Contergan as a sedative. The early symptoms of the ill effects of Thalidomide is a prickling feeling of the extremities followed by a numbness and coldness. This usually begins in the toes and is not initially noticed or obvious to the patient. The numbness slowly spreads to the ball of the foot, then to the ankles and finally to the calves. It is months before numbness appears in the tips of the fingers, and much more time elapses before toxic polyneutitis develops bringing severe muscular cramps and weakness of the limbs. All these symptoms take place before damage to the central nervous system is visually noticeable. After a further lapse of time the patient loses coordination, and, unable to judge the position of his legs becomes unbalanced, suffers twitchings of the facial muscles, trembling of the muscles of the entire body, speech difficulty, double vision, and, in some cases, epileptic seizures. How, we ask the producers of ARSL, could the use of more animals reveal the early symptoms of nerve damage which is not even noticeable to the HUMAN patient? Further the human being is capable of communicating his dis-ease and distress, but how are laboratory animals supposed to report tingling in the limbs and tail, inability to concentrate, onset of double vision, slurred speech etc, bearing in mind that before the onset of visual problems have time to eventuate the animals' short life-span is finished?
Though it is illogical to blame medical tragedies like the Thalidomide disaster on insufficient animal-testing, it is extremely logical and understandable that the publishers of ARSL who are all involved in vivisection do so, for to do otherwise would be to advocate the necessity for a change of direction to conclusive methods of drug testing which would expose their various institutions as redundant - if not downright fraudulent.
The decision to use animals to test the new wave of wonder-drugs was a fortuitous one for the drug manufacturers with its water-tight alibi of inconclusiveness, and hingeing upon this factor a battery of new businesses mushroomed as animal-breeders and other ancillary industries thrived. Others basking in the rich pickings were (and are - even more so today) holders of shares in these companies, and they, like the producers of ARSL, are unlikely to kill the horse that carries them to the bank by admitting it's heading in the direction of profit, not health. Whilst the dividends pour in it is extremely unlikely that the financial beneficiaries of vivisection lose sleep because vivisection is a fundamental error which kills animals and people, for it represents a source of income which would cease immediately should animal experiments be abolished.
Thus the machinery was set up to support the quick development of drugs for all our problems. Drugs to give us freedom from stress, to make us sleep, to wake us up, to prolong our youth and, in the case of Thalidomide, to sedate, tranquillise and give us serene pregnancies without nausea. The only dark cloud on the sunny sea of profits being when they are interrupted with irritating set-backs as tragedies strike, as they do, from time to time, with increasing regularity.
(Further details refer to Chapter 21, Section 3 - Drugs and the Law.)
Whilst on the subject of the new era of pharmaceuticals and instant health without responsibility, two further important factors must be taken into account.
- The development of modern pharmacological science within the privately-owned industrial and pharmacological industries could never have flourished without the involvement of governmental and academic institutions. Throughout the world, the universities, hot-beds of vivisection are supported by pharmaceutical companies and by government funds. Most big hospitals have their underground vivisection hell-holes which are paid for by the taxpayer, as are the huge government grants given to the vivisectors. Conversely some drug companies receive aid from the State. The writer believes that it is fair to assume that this web of inter-connected interests accounts for the blank looks, stony silence and hostility which betrays the politicians when they are approached on the subject of vivisection. It is significant that the results of these partnerships inevitably focus on the lucrative chemical and artificial treatment of disease and its associated profits rather than on prevention and education.
- The recent development of batteries of new pharmaceuticals has not been uncriticised. In fact there are many recorded warnings, for example:
- In a lecture delivered in October 1963 in San Francisco, Dr Louis Lasagna, a member of the staff of the Johns Hopkins University School of Medicine, Baltimore gave results of a random survey of one hundred patients revealing that seven percent were suffering from doctor-prescribed drug reactions. He warned that a considerable length of time would elapse before the medical profession became fully aware of the troubles that drugs can cause. The same Dr Lasagna went on to write in Science, a journal with a worldwide circulation - on December 5 1969:
"Paradoxically medical care has in some ways been impaired by the availability of new medicaments and of confusion regarding the diagnosis of disease because of the temptation to treat symptoms without having determined their cause. A fully effective attack on the major health problems facing the public requires not only new basic information concerning such diseases as artherosclerosis, cancer, arthritis and schizophrenia - but preventive measures instead of therapeutic manoeuvres."
- In 1963 Dr E.M. Schimmel in the Journal of Chronic Diseases analysing the problems of major toxic reactions and accidents in a university hospital in the U.S.A. found that approximately one patient in every ten suffered from some kind of drug-induced disease. Clinical Pharmacology and Therapeutics runs a section on the high frequency of reported drug damage saying it constituted merely the floating tip of an iceberg - that the major extent of drug damage remains hidden. This is extremely fortunate for the drug companies, if not for the patients, as death or damage arising from drug damage can be attributed to other causes through accidental or deliberately manoeuvred incorrect diagnoses. Or it can be ignored altogether, for example:
From 1947 to 1951, 79 children in the University Paediatric Clinic of Hamburg had been treated with the drug Spirocid for congenital syphilis. Twelve developed severe encephalitis (brain damage) resulting in permanent severe damage of the nervous system. Though the drug was confirmed as the factor causing the damage the drug company Hoechst continued to dispense and sell it for a further eight years - even recommending it "to be taken twice daily as a tonic".
Though three decades have elapsed we should be heeding the words of these and a new generation of doctors who are warning that the erroneous reliance on animal studies is responsible for drug damage which is now causing more problems to the human race than any other single factor - with the exception of its annihilation by nuclear war. Dr Robert Sharpe, Medical Adviser, NAVS, London now puts the figure of people in hospitals directly due to the toxic effects of drugs at 16 percent and this is confirmed by other doctors.
But animal testing was the chosen method of testing the drug Thalidomide prior to prescribing it to pregnant women!
"Thalidomide... Was this just an innocent case of a tranquilliser turning out to have monstrous side-effects on children to be born? Or was something uglier at work in its destructive career?"
So reads the preface of the Penguin Special titled : Thalidomide and the Power of the Drug Companies. Published in 1972. Written by two Swedes:
- Lawyer, Henning Sjostrom. Acts for people injured or damaged by medical preparations including many Thalidomide victims. Is currently concerned with the implications of oral contraceptives;
- Research Chemist, Robert Nilsson. Principal scientific adviser and technical coordinator of the Thalidomide trials in Scandinavia on behalf of the plaintiffs as well as technical adviser for the prosecution in Aachen, Germany.
These investigators claim that Thalidomide was KNOWN to be dangerous for the damage it could do to the nervous system before it was put on the market. Similarly, that the threat the drug represented to unborn babies was KNOWN before it was withdrawn.
Recalling the legal battles which were fought around Thalidomide in Western Europe, the U.S.A., Japan and Australia, the authors quote from their findings and memoranda. They strongly suggest that the mysteries of science may place too much power in the hands of those who are out for profits. The following information is taken from the evidence exposed during the Thalidomide trials:
In the 1950s at the University Clinic at Bonn, Thalidomide had been tested on 140 children, seven of whom were less than a year old. Forty children, most of whom had brain damage, had been given the drug for up to nine weeks. The parents were not asked for their permission, nor were they informed that their children were being treated with an entirely new sedative. Doses used were 11 to 20 times higher than the recommended dose for adults. Half the children were mentally disturbed or had brain damage. Other children also received Thalidomide in the same high dosage. One child had a circulatory collapse, one died from a congenital heart defect, a three-month-old baby died from heart failure, a twentyone-month-old baby temporarily lost her vision. The doctor responsible stopped using the drug when he heard that his medical colleagues had similar experiences with Thalidomide. (Twelve years were to elapse before Thalidomide was withdrawn from the market.)
In 1955, one year before the commencement of the marketing of Thalidomide in its various formulae, three physicians, along with a Professor Kloos, took part in a symposium arranged by Chemie Grunenthal at which they reported to the company unsatisfactory experiences with Thalidomide. These were ignored!
In 1956 the drug giants (then) SmithKline and French (now SmithKline Beecham) revealed that even when used in very high doses Thalidomide could not induce sleep in mice. When administered at doses 50 times larger than that claimed by Chemie Grunenthal to be "sleep inducing" this company could still not achieve the hypnotic effect in animals that it had on man. Nor when given 650 times the dose effective in man. This was substantiated and confirmed at the trial by drug companies Richardson-Merrel and Ciba.
In November 1956 and October 1957 Thalidomide was marketed in Germany by Chemie Grunenthal. Sales rocketed as many pharmaceutical companies produced the drug under license to Chemie Grunenthal from as early as 1955 and 1957. Simultaneously through these years a succession of clinical investigators, through observation of their patients, reported adverse effects of Thalidomide. The pharmaceutical company (then) SmithKline and French reported ill-effects from the drug and the same problems were confirmed by doctors observing their patients through to 1959. Chemie Grunenthal minimised the reports by ascribing them to overdosage and prolonged usage. Then followed a surge of universal medical agreement that severe nervous damage was being caused by Thalidomide.
In 1958 Chemie Grunenthal sent the following letter to 40,000 doctors:
"In pregnancy and during the lactation period the female organism is under great strain. Sleeplessness, unrest and tension are constant complaints. The administration of a sedative and a hypnotic that will hurt neither mother nor child is often necessary."
(This was to encourage gynaecologists and obstetricians to prescribe Contergan and Contergan Forte (Thalidomide) to patients.)
On August 27 1959 one of the Chemie Grunenthal partners in Basel gave the following report on the situation in Switzerland:
"Twenty well-known physicians have now informed our public relations men that they themselves, or their patients, have still had severe side-effects the morning after taking one whole tablet of Softenon Forte (Thalidomide) in the form of extreme tiredness, tremor (shaking) of the hands etc. Professor Ludwig, head doctor of the second medical section of Burgerspital, Basel, added: 'Once and never again. This is a horrible drug.'"
In September 1959 the use of Contergan (Thalidomide) was stopped in a German Hospital because of severe reactions.
On November 3 1959 a written report was received by Chemie Grunenthal from a neurologist Dr Ralf Voss of Dusseldorf reporting more adverse effects. Dr Voss asked if Thalidomide could cause damage to the peripheral nervous system. Chemie Grunenthal replied that such effects had never been observed before. At the trial this statement was proved false.
In December 1959 Dr Somers, a scientist in Distillers laboratory (Chemie Grunenthal's licensee in England), reported grave doubts about the safety of Thalidomide which was being widely advertised as a very safe drug. In an internal report Dr Somers wrote:
"Hitherto thalidomide has shown no demonstrable toxicity and mice have survived oral doses as high as 5g/kg... The observations that our formulated suspension is toxic is disturbing for it means that if we market in this form our claims are no longer justified and it is suggested that the formulation is amended to avoid this situation."
On January 2 1960 Dr Somers again expresses alarm:
"The fact that it [Thalidomide] can be toxic is the worry. You will appreciate that our claim for non-toxicity would not be valid with this preparation."
In reply Chemie Grunenthal informed Distillers that they had repeated Dr Somers' experiments and had arrived at the conclusion that the preparation was completely non-toxic to the mice used at Chemie Grunenthal, and that the British mice they had used must belong to some particularly sensitive strain.
At the trial it was acknowledged that animals reacted quite differently from man, that the apparent safety observed in animals would be absolutely no guarantee that this would be applicable to man, as was indeed proved correct. It was also disclosed at the trial by Dr Muckter, the director of the scientific laboratory of Chemie Grunenthal, that all the company's records were destroyed - or had "disappeared" during 1959!
By 1960 sales of Thalidomide were stepped up, despite reports of malformations caused by the drug which now poured in from all over the world. It was now being marketed by 14 firms in many countries under 37 different trade names and sold without prescription. It was combined with other drugs like aspirin and prescribed widely for headaches, migraine, coughs, colds, flu, asthma, neuralgia, nervous debility, to quieten frisky babies and to give pregnant women a good night's sleep. Globally Thalidomide was the big winner which dominated prescriptions. The British pharmaceutical company Distillers produced Thalidomide for morning sickness where it was distributed throughout the British Isles, Australia and New Zealand under the trade name of Distavel, the advertisement reading:
"Distavel can be given with complete safety to pregnant women and nursing mothers without adverse effect on mother or child... Outstandingly safe Distavel [Thalidomide] has been prescribed for nearly three years in this country."
Throughout Britain, Australia and New Zealand it was also prescribed as a tranquilliser under the trade names: Valgis, Tensival, Valgraine and Asmavel.
In April 1961 Australian Dr W.G. McBride at Crown Street Women's Hospital, Sydney, notified the representatives of Distillers in Australia about his suspicions of the link between Distavel (Thalidomide) and malformations. Distillers in England claim they never received the written report. Sales promotion of the drug was stepped up and a quarter of a million leaflets distributed saying Thalidomide is "Harmless even over a long period of use" and "completely harmless even for infants".
May 4 1961 Dr McBride reported further malformations due to Thalidomide and succeeded in convincing his superiors that the drug must be withdrawn from use in the hospital. In October and November Dr McBride reported further malformed babies.
November 27 1961 Thalidomide was withdrawn from the British market.
December 16 1961 Dr McBride's observations were published in the Lancet, and in the Australian Medical Journal on December 23.
On January 6 and February 3 1962 Prof. Widijung Lenz who had warned against Thalidomide in Germany published evidence of deformities in Lancet. Chemie Grunenthal continued prescribing Thalidomide, stepping up its advertising and intensive marketing despite criticism of doctors.
March 4 1962 Thalidomide was removed from the shelves in Germany because of public opinion and against the wishes of Chemie Grunenthal. News of the dangers of Thalidomide was played down by the media. In many cases malformed births occurred after the drug was withdrawn as, in possession of the drug mothers took it never realising the risks involved. At the time of withdrawl of Thalidomide in Germany thousands of malformed babies had been born, thousands of women required extensive psychiatric treatment and there were many suicides. (In some countries Thalidomide continued to be prescribed and was doled out to pregnant women in Canada until August 1962.)
It is not the writer's intention to dwell on the hideous and hitherto unknown malformations that Thalidomide caused to babies, or the vast number of victims which in their thousands can never be precisely assessed in the official casualty figures as in many of the poorer countries "monster babies" and "freaks" were locked from view or destroyed by distraught parents who accepted the terrible afflictions as visitations from the devil. Rather, she attempts to bring the readers' attention to the pharmaceutical industry's callous attention to profit and its complete indifference to suffering which is spot-lighted as one researches the records of the Thalidomide trials. Before continuing it is important that the following points are recapitulated:
- Clinical investigators who had closely monitored their patients for years were ignored as they reported Thalidomide damage to Chemie Grunenthal. Clinical investigators tabulated adverse effects from the early 1950s. The drug was not withdrawn until 1962.
- The fault of doctors cannot be over-emphasised for the Thalidomide tragedy and lessons should have been learned from their negligence as they blindly accepted the assurance of the drug company that the product was safe. Thus they were exposed as mere pill-pushers. (When one considers the incentives offered to doctors by drug companies in exchange for prescribing their products this is hardly surprising.)
- Doctors again must be hauled up for censorship for in prescribing Thalidomide to pregnant women they flouted the cardinal rule of medicine that drugs are dangerous to pregnant women and should not be prescribed. For example:
- "In the ten years preceding the appearance of Thalidomide not less than 25 compounds were shown by various investigators ranging from Japan to the United States, to England and France, to affect the foetus in utero, either killing many foetuses or inducing malformations. The findings by the various investigators were published in scientific journals and distributed internationally."
Prof. John B. Thiersch, Director of the Institute of Biological Research and Professor of Clinical Pharmacology in the University of Seattle - in a statement at the Canadian Thalidomide Hearings.
- "I want to emphasize that the patients took Contergan for at the most eight to ten days. This drug was never prescribed for pregnant women. It is my absolute principle never to give sleeping pills or tranquillisers to a mother-to-be. It is an old rule in medicine that basically, no barbiturates, opiates, sedatives or hypnotics should be given to mothers-to-be because these drugs can affect the foetus."
Dr Blasiu before the prosecutor in Aachen on June 5 1964 giving testimony concerning his clinical trials on which Chemie Grunenthal and other distributors of Thalidomide had based their advertisements.
- "For some thirty years or more I have been warning against the free and unwarranted use of drugs (any drug) during pregnancy. That is long before the tragic Thalidomide disaster. For decades I have been teaching my medical students to exercise the utmost care in the prescribing of drugs to pregnant women... In the enclosed article you will notice that already I sounded a warning note about the dangers of the administration of drugs to expectant mothers... The greatest danger to the foetus exists during the first trimester of pregnancy. I have for years warned especially against the administration of any drug to pregnant mothers."
"Any firm manufacturing pharmaceutical preparations should for decades have been fully aware of the possible dangers of drugs to pregnant mothers and foetuses."
In his deposition to the Court on the Thalidomide Hearings, Professor Duow G. Steyn at the Ministry of Health in the Republic of South Africa.
- "There has been almost completely ignored certain longstanding evidence to the effect that chemical agents can cause injury to babies in their mothers' wombs."
Senator Hubert Humphrey in the Thalidomide Hearings before the U.S. Senate in 1962.
But a more critical and greater universally-recognised fact had been ignored in the production of Thalidomide - of which the serious anti-vivisectionists will not need to be reminded:
The safety of the drug had been based on the assumption that the principle of testing on animals is bona fide. A fact known to be incorrect as we are constantly reminded by the increasing number of honest doctors who are speaking out as follows:
- The effect of a drug varies in different species of animal and in animals of the same species.
- The effects evoked in animals differ from those it creates in human beings.
- Many diseases occurring in man cannot be induced in experimental animals, and certain diseases develop quite differently in animals.
More evidence of the avarice of drug companies came up at the Thalidomide trial when clinical investigator Dr Jung who had received regular monthly payments for testing Grunenthal drugs stated that Contergan had proved to be an excellent sedative. When questioned at the trials about a report in which he had written that he had "stopped administration" of the drug in three cases because of side-effects, Jung declared that the expression "stopped administration" was badly chosen. In fact he had merely reduced the dose. During an hour-long interrogation of Jung the latter declared: "I think you are under the impression that medicine is an exact science. You are quite wrong. A doctor has to take many decisions on the basis of experience and intuition." (And on the basis of financial encouragement received from the drug companies - Author.)
- "In approximately 10 strains of rats, 15 strains of mice, eleven breeds of rabbit, two breeds of dogs, three strains of hamsters, eight species of primates and in other such varied species as cats, armadillos, guinea-pigs, swine and ferrets in which Thalidomide had been tested, teratogenic effects (birth defects) have been induced only occasionally."
(J.L. Schardein, "Drugs as Teratogens", Outrage, No. 77, Dec/Jan 1992.)
- "The high therapeutic activity of many of the new drugs render them dangerous in use and liable to produce undesirable side-effects."
The Director of the Swedish Medical Board, Dr Engel representing Sweden at the World Health Organisation in May 1962. On March 4 1962 Thalidomide had been removed from the market.
- "It is estimated that in 1977... 120,366 patients were discharged from or died in hospital in the U.K. due to the adverse effects of medicinal agents."
(Sir George Young, Health Minister 1980, Outrage No. 66, Feb/Mar 1990.)
- "The problem arises out of the fact that they (the drug companies) market so many of their failures... but I should point out that with many of these products it is clear while they are on the drawing board that they promise no utility. They promise sales."
(Dr A. Ball Console, previous medical director of Squibb addressing the Subcommittee on Antitrust and Monopoly, 1955.)
NOTA BENE
On June 22 1971 Australian Dr William McBride, internationally respected scientist and gynaecologist was invited to Paris by the Institut de la Vie where 18 Nobel Prize winners gathered to pay tribute to the man who alerted the world to the dangers of Thalidomide. In a ceremony which he described as "the most shimmering moment of glory never to be forgotten" he was presented with a gold medal and cash prize of $40,000 (with which he established an institute for study of the first 41 weeks of life). In 1969 he was made a Commander of the British Empire and in 1977 awarded the Order of Australia. (By the time Thalidomide was withdrawn thousands of babies had been born with terrible malformations, including 27 in Australia.)
In 1972 after being named Father of the Year, Dr McBride created another international furore by announcing that Imipramine, a widely-used anti-depressant, caused birth deformities. (The Australian Drug Evaluation Committee rejected the evidence.) In 1980 turning his attention to the Debendox anti-nausea drug produced by Merrel Dow he appeared as an expert witness for the U.S. women who had children with birth defects and were sueing the manufacturer. The drug was subsequently removed from the market. There were many Debendox victims in Australia and New Zealand. Then McBride said his research showed that Debendox was also responsible for causing mental retardation. On December 12 1987 McBride was accused of scientific fraud, of manoeuvring the results of the Debendox experiments. In November 1988 he was found guilty by a committee of inquiry and appealed against the decision. Dr McBride says that everyone will eventually realise that he is innocent of the charges against him. In an interview with Jane Cadzow, senior writer for the Australian Good Weekend he accused the N.S.W. Health Department of being a "Gestapo State". "There's big money behind this", he said: "You know, big business is just as vicious as the CIA. Because I've given evidence for the kids in America... The drug companies have been known to resort to drastic methods to discredit those who appear in court against them." Dr McBride launched a Supreme Court action in an attempt to overturn the findings brought against him. (Little wonder doctors are hesitant to swim against the tide of conventional established thought by asking for the abolition of vivisection, the cause of drug failures - Author.)
(Good Weekend, The Sydney Morning Herald Magazine, July 15 1989.)
The frequency of the epidemiologically occurring malformations in Germany followed the absolute sales of Thalidomide with a time-lag of a little less than one year (see the figure below). Eight to nine months after the withdrawal of Thalidomide from the market the wave of typical malformations disappeared as suddenly as they had appeared, after the same time-lag as followed the introduction of the drug.
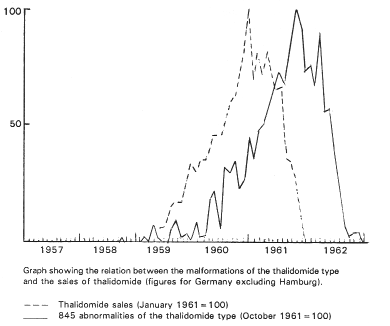 |
(Hennina Sjostrom and Robert Nilsson, Thalidomide and the Power of the Drug Companies, page 156.)
- "The folly of founding the actions of drugs on animal experiments cannot be over-emphasized."
(Editorial, Medical Review, September 1953.)
- "In practise, all animal experiments are scientifically indefensible, as they lack any scientific validity and reliability in regard to humans. They only serve as an alibi for the drug manufacturers, who hope to protect themselves thereby."
(Dr H. Stiller and Dr M. Stiller, Tierversuch and Tierexperimentator, Hirthammer Verlag, Munich, 1976.)
As written in a footnote in Chapter 21 Drugs and the Law, Dr McBride, though opposing his colleagues, had no anti-vivisection tendencies. Rather he was a fully-fledged vivisector. At its peak his Foundation 41 employed 16 researchers. The laboratories are now empty, the meeting rooms closed, and for his insistence that drugs are dangerous to the unborn child, a fact that abolitionist doctors warn without recourse to animal experiments, Dr McBride is isolated and persecuted.
TRADE IN MISERY
Another twist to the story took place on Tuesday April 27 1992 when a consignment of 200 marmosets, monkeys which are highly social animals prone to multiple births and therefore favoured by the vivisectors researching reproductive biology, left Australia on board a British Airways flight bound for London. Purchased from Foundation 41 by Charles Rivers Ltd, the world's largest laboratory animal breeder and supplier, the animals were transported in wooden crates 1'x1'x 3', this small space being subdivided into four sections each containing two monkeys. After suffering 40 hours of indescribably stressful travel, witnesses when lifting the cage covers saw there was no water provided and say they could hear the monkeys uttering clearly distinguishable distress calls. On arrival in London they were switched from a British Airways lorry to the vehicle of the Charles Rivers company and transferred to Margate. Here they await sale to British or European vivisection laboratories where horrific experiments await them - or alternatively could be sold to the Dutch Ministry of Defence which has previously requested Charles Rivers to supply them with marmosets for use in chemical warfare research.
(Outrage, August/September 1992.)
Nota-Bene
In August 1992 due to pressure from anti-vivisectionists British Airways banned the carriage of live monkeys except for conservation. Effectively this takes the airline out of the international trade in monkeys for vivisection laboratories.
<< previous page | next page >>
contents | index
