| June 2006 http://www.seruminstitute.com/ |
|
|||
|
|
 |
|
||
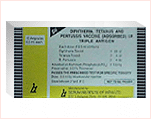
DESCRIPTION
Diphtheria, Tetanus and Pertussis Vaccine (Adsorbed) (Sii Triple
Antigen) as supplied by Serum Institute of India Ltd. is a sterile,
whitish turibid, uniform suspension of diphtheria, tetanus toxoids and
pertussis vaccine adsorbed on aluminium phosphate and suspended in
isotonic sodium chloride solution.Each dose of 0.5 ml contains:
| Diphtheria Toxoid |
|
| Tetanus Toxoid |
|
| B. Pertussis |
|
|
Adsorbed on Aluminium Phosphate (AlPO4)
|
|
| Thiomersal | 0.01% as preservative. |
INDICATIONS
DPT Vaccine (Adsorbed) is indicated for the primary immunization of
infants, at or above the age of 6 weeks, and of children through six
years of age against diphtheria, tetanus and whooping cough.
DOSAGE
For the purpose of primary immunization it is recommended that 3 doses
each of 0.5 ml should be inoculated on 3 separate occasions at 4 weeks
interval.
The first dose should be given at approximately 6 weeks of age.
Reinforcing injections of 0.5 ml should be given 12 months after the
primary immunization and also between the ages of 4 to 6 years.
Although it is recommended that immunization be started at 6 weeks, if
for any reason it is delayed, the same schedule may be used up to the
sixth birthday.
A reinforcing injection of 0.5 ml. intramuscularly should be
administered between four and six years of age (i.e. at the time of
school entry).
This booster dose is not necessary if the fourth primary immunizing dose
has been administered after the fourth birthday.
ADMINISTRATION
DPT vaccine should be administered by deep intramuscular injection. The
preferred site for injection is the anterolateral aspect of the upper
thigh.
Only sterile needles and syringes should be used for each injection. The
vaccine should be well shaken before use.
Each injection of the primary immunization series should be made into a
different site with a sterile disposable syringe and needle.
ADVERSE REACTIONS
Mild local reactions consisting of erytherna, pain and tenderness,
swelling and induration at the injection site are common, usually
self-limited and subside without treatment. Persistent nodules at the
site of injection have occurred following the use of an adsorbed
vaccine, but this complication is unusual. Abscess at the site of
injection has been reported (6-10 per million doses).
Mild to moderate systemic reactions occur frequently following
injections of this vaccine. These usually consist of one or more of the
following symptoms and signs: temperature elevation 38°C, drowsiness,
fretfulness, anorexia, vomiting, irritability, persistent or unusual
crying. These symptoms are most frequent during the first 24 hours
following vaccine injection and may persist for one to two days. The
following adverse reactions -high fever (40.5°C), collapse, screaming
episodes, convulsions, signs of encephalopathy which can be serious and,
occasionally fatal have been reported following administration of
preparations containing pertussis vaccine. The incidence of these
reactions is unknown, but they seem to be exceedingly rare, if any of
these reactions occur, further immunization against pertussis is
contraindicated. See also Contraindications and Precautions.
Sudden-infant-death-syndrome (SIDS) has been reported following
administration of vaccine containing diphtheria, tetanus toxoids and
pertussis vaccine. The significance of these reports is not clear. It
should be borne in mind that the three primary immunizing doses of these
vaccine are usually administered to infants between the age of 6 weeks
and 6 months and that approximately 85% of SIDS cases occur in the
period from one through six months of age with the peak incidence at age
two to four months.
CONTRAINDICATIONS
DPT Vaccine (Adsorbed) should not be administered to infants or children
with high fever, or other evidence of acute illness or infection. The
presence of an evolving or changing neurological disorder is a
contraindication to receipt of this vaccine. While data to support
exclusion of pertussis immunization because of a family history of
convulsive or other neurological disorders are scarce, a personal or
family history of central nervous system disease or convulsions is
considered a contraindication to use of this vaccine. Occurence of any
of the following signs, symptoms or conditions following administration
is a contraindication to further use of this product and or pertussis
vaccine as the single antigen: fever over 40°C (104°F); convulsion(s)
with or without accompanying fever; alterations of consciousness; focal
neurologic signs; screaming episodes; shock; collapse; thrombocytopenia
purpura.
DPT Vaccine (Adsorbed) should not be administered to children over six
years of age or to adults because of the danger of reactions to
diphtheria toxoid or to pertussis vaccine and because pertussis is less
severe in these age groups than in infants and young children.
The specific contraindications adopted by individual national health
authorities should reflect a balance between the risk from the vaccine
and the risk from the disease. Because the risk from the vaccine remains
extremely low in comparison to the risk from the disease in many
developing countries, authorities there may choose to offer immunization
to children who are mildly to moderately ill or malnourished.
PRECAUTIONS AND WARNING
Individuals receiving corticosteroids or other immunosuppressive drugs
may not develop an optimum immunologic response. This product should be
used only for infants and children from 6 weeks through six years of
age. The possibility of allergic reactions in individuals sensitive to
the components of the vaccine should be borne in mind. Adrenaline
injection (1:1000) should be kept ready for immediate use in case an
anaphylactic or acute hypersensitivity reaction occurs. Frequent booster
doses of tetanus toxoid in the presence of adequate or excessive serum
levels of tetanus antitoxin have been associated with increased
incidence and severity of reactions and should be avoided. If
hypersensitivity to the diphtheria component is suspected, tetanus
toxoid should be used for reinforcing doses.
A separate sterile syringe and needle should be used for each individual
patient to prevent the transmission of hepatitis or other infectious
agents.
DPT VACCINE (ADSORBED) SHOULD BE USED ONLY FOR INFANTS AND CHILDREN
THROUGH SIX YEARS OF AGE.
WITHDRAWING THE VACCINE FROM A SEALED GLASS AMPOULE
Shake the ampoule to disperse the contents thoroughly immediately before
withdrawing the dose.Tap the ampoule to ensure that the solution is in
the lower portion rather than in the neck of the ampoule Wipe the neck
of the ampoule with a suitable antiseptic using a sterile piece of
cotton break off the top of the ampoule at the constriction by thumb
pressure.
WITHDRAWING THE VACCINE FROM A RUBBER-STOPPERED VIAL
DO NOT REMOVE THE RUBBER STOPPER FROM THE VIAL
Shake the vial to disperse the contents thoroughly immediately before
each withdrawal of vaccine. Apply a sterile piece of cotton moistened
with a suitable antiseptic to the surface of the rubber stopper and
allow to dry. Draw into the sterile syringe a volume of air equal to the
amount of vaccine to be withdrawn from the vial. Pierce the centre of
the rubber stopper with the sterile needle of the syringe. invert the
vial, slowly inject into it the air contained in the syringe, and
keeping the point of the needle immersed. withdraw into the syringe the
required amount of vaccine. Then hold the syringe plunger steady and
withdraw the needle from the vial.
Carefully insert the needle intramuscularly at the prepared injection
site. In order to avoid intravenous injection, pull back the plunger of
the syringe to make certain that no blood is withdrawn before injecting
the desired dose.
STORAGE
Diphtheria, Tetanus and Pertussis Vaccine (Adsorbed) should be stored at
a temperature between 2°C and 8°C (35° to 46°F).
NOT TO BE FROZEN.
Product which has been exposed to freezing should not be used.
PRESENTATION
Diphtheria, Tetanus and Pertussis Vaccine (Adsorbed) is supplied, ready
for use, in rubber-stoppered multi-dose vials, and in single-dose glass
ampoules.
i) 0.5 ml X 10 ampoules box
ii) 0.5 ml X 50 ampoules box
iii) 5 ml - 10 dose single vial carton
iv) 5 ml - 10 dose X 50 vials box
|
|
|||||||||||||||||||||||||||||||||||||||||||||||
COMPOSITION Each ml contains :
In areas of intermediate or high prevalence
of hepatitis B, with most of the population at risk of
acquiring the disease, immunisation should be offered to all
neonates and young children. Immunisation should also be
considered for adolescents and young adults.
CONTRAINDICATIONS
Gene Vac-B™ should not be
administered to subjects with known hypersensitivity to
any component of the vaccine, or to subjects having
shown signs of hypersensitivity after previous Hepatitis
B Vaccine administration.
ADVERSE REACTIONS
The immunisation schedule may be adapted to meet local
immunisation recommendations.
The following timing of injections gives general guidance :
SPECIAL DOSAGE RECOMMENDATIONSDOSAGE
RECOMMENDATION FOR NEONATES BORN OF MOTHERS WHO ARE HBV
CARRIERS. METHOD OF ADMINISTRATION STORAGE
|
||||||||||||||||||||||||||||||||||||||||||||||||



